Abstract
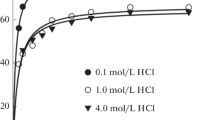
Extraction of palladium(II) with diacylated triethylenetetramine hydrochloride (with chloroform as diluent) from hydrochloric acid solutions was studied. Palladium(II) extraction from 3 mol/L HCl solutions occurs via anion-exchange mechanism. Concentrational constants were calculated and thermodynamic parameters of extraction were estimated.
Similar content being viewed by others
References
I. Szczepanska, A. Borowiak-Resterna, and M. Wisniewski, Hydrometallurgy 68, 159 (2003).
H. Narita, K. Morisaku, and N. Tanaka, Chem. Commun., No. 45, 5921 (2008).
N. G. Afzaletdinova, Yu. I. Murinov, M. Yu. Bibikova, et al., Russ. J. Inorg. Chem. 49, 955 (2004).
S. O. Bondareva, N. G. Afzaletdinova, and Yu. I. Murinov, Russ. J. Appl. Chem. 84, 1897 (2011).
L. G. Golubyatnikova, G. R. Anpilogova, S. O. Bondareva, et al., Russ. J. Gen. Chem. 83, 624 (2013).
R. A. Khisamutdinov, S. O. Bondareva, Yu. I. Murinov, and I. P. Baikova, Russ. J. Inorg. Chem 53, 462 (2008).
S. O. Bondareva, Yu. I. Murinov, and V. V. Lisitskii, Russ. J. Appl. Chem. 80, 279 (2007).
E. G. Ginzburg, N. Z. Ezerskaya, S. M. Prokof’eva, et al., Analytical Chemistry of Platinum Metals (Khimiya, Moscow, 1972) [in Russian].
V. I. Slesarev, Chemistry: Fundamentals of Life Chemistry. Textbook for Higher Education Institutes (Khimizdat, St. Petersburg, 2005) [in Russian].
J. R. Ferraro, Low-Frequency Vibrations of Inorganic and Coordination Compounds (Plenum Press, New York, 1971).
A. B. P. Lever, Inorganic Electronic Spectroscopy (Amsterdam, Elsevier, 1984).
Yu. N. Kukushkin, Chemistry of Coordination Compounds (Vysshaya shkola, Moscow, 1985) [in Russian].
Comprehensive Analytical Chemistry, Vol. 6 (Elsevier, Amsterdam, 1976).
D. M. Adams, Metal-Ligand and Related Vibration: A Critical Survey of the Infrared and Raman Spectra of Metallic and Organometallic Compounds (Edward Arnold, 1966), Vol. 1, p. 60.
V. V. Belova, T. I. Jidkova, and S. A. Vasilevich, Solv. Extr. Ion Exch. 15, 1023 (1997).
V. V. Belova, T. I. Zhidkova, and A. I. Khol’kin, Zh. Neorg. Khim. 39, 656 (1994).
V. P. Vasil’ev, Analytical Chemistry: Gravimetric and Titrimetric Analysis (Vysshaya shkola, Moscow, 1989), Vol. 1 [in Russian].
H. A. El-Boraey, Spectrochim. Acta, Part A 97, 255 (2012).
T. M. Silva, S. Oredsson, L. Persson, et al., J. Inorg. Biochem. 108, 1 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.G. Golubyatnikova, R.A. Khisamutdinov, S.O. Bondareva, A.N. Lobov, Yu.I. Murinov, 2014, published in Zhurnal Neorganicheskoi Khimii, 2014, Vol. 59, No. 6, pp. 801–807.
Rights and permissions
About this article
Cite this article
Golubyatnikova, L.G., Khisamutdinov, R.A., Bondareva, S.O. et al. Palladium(II) extraction from hydrochloric acid solutions with diacylated triethylenetetramine. Russ. J. Inorg. Chem. 59, 620–625 (2014). https://doi.org/10.1134/S0036023614060084
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023614060084