Abstract
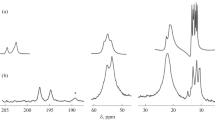
The interaction between cadmium cyclo-pentamethylenedithiocarbamate (chemisorbent Ia) and the [AuCl4]− anion in 2 M HCl has been investigated. The state of the chemisorbent in contact with AuCl3 solutions has been probed by 113Cd MAS NMR spectroscopy. The heterogeneous reactions in the system, including gold(III) chemisorption from the solution and partial ion exchange, yield the gold(III)-cadmium heteropolynuclear complex ([Au{S2CN(CH2)5}2]2[CdCl4]) n (I) and the polynuclear mixed-ligand complex ([Au{S2CN(CH2)5}Cl2]) n (II). The crystal and molecular structures of these compounds have been determined by X-ray crystallography. The main structural units of the compounds are the complex cation [Au{S2CN(CH2)5}2]+, [CdCl4]2− anion (in I), and Au{S2CN(CH2)5}Cl2 molecule (in II). The further structural self-organization of the complexes at the supramolecular level is due to secondary Au...S and Au...Au bonds. [Au2{S2CN(CH2)5}4]2+ dinuclear cations form in the structure of I, which then polymerize into ([Au2{S2CN(CH2)5}4]2+) n chains. In the structure of II, adjacent Au{S2CN(CH2)5}Cl2 molecules join by forming pairs of asymmetric secondary Au...S bonds, producing polymer chains with alternating antiparallel monomer units. The chemisorption capacity values calculated for cadmium cyclo-pentamethylenedithiocarbamate from gold(III) binding reactions are 455 and 910 mg of gold per gram of sorbent. The gold recovery conditions have been determined by investigating the thermal behavior of I and II by synchronous thermal analysis. The multistep thermal destruction of ionic complex I includes the thermolysis of its carbamate moiety and [CdCl4]2− (which liberates gold metal and cadmium chloride and yield some amount of CdS) and CdCl2 and CdS evaporation. The thermolysis of II proceeds via the formation of Au2S and AuCl as intermediate compounds. In both cases, the ultimate pyrolysis product is elemental gold.
Similar content being viewed by others
References
D. V. Konarev, A. Y. Kovalevsky, S. S. Khasanov, et al., Eur. J. Inorg. Chem., No. 9, 1881 (2006).
B. Arulprakasam, K. Ramalingam, G. Bocelli, and A. Cantoni, Polyhedron 26(15), 4489 (2007).
S. Thirumaran, K. Ramalingam, G. Bocelli, and L. Righi, Polyhedron 28(2), 263 (2009).
P. V. Subha, P. Valarmathi, N. Srinivasan, et al., Polyhedron 29(3), 1078 (2010).
T. A. Rodina, A. V. Ivanov, A. V. Gerasimenko, et al., Inorg. Chim. Acta 368(1), 263 (2011).
Dulare Ram, M. K. Bharty, A. Singh, and N. K. Singh, Polyhedron 31(1), 373 (2012).
K. S. Siddiqi, S. A. A. Nami, Lutfullah, and Y. Chebude, J. Braz. Chem. Soc 17(1), 107 (2006).
S. Khan, S. A. A. Nami, and K. S. Siddiqi, J. Mol. Struct. 875(1–3), 478 (2008).
D. C. Onwudiwe and P. A. Ajibade, Polyhedron 29(5), 1431 (2010).
P. A. Ajibade, D. C. Onwudiwe, and M. J. Moloto, Polyhedron 30(2), 246 (2011).
R. Marx Nirmal, K. Pandian, and K. Sivakumar, Appl. Surf. Sci. 257(7), 2745 (2011).
A. V. Ivanov, S. A. Zinkin, V. I. Sergienko, and A. V. Gerasimenko, Russ. J. Coord. Chem. 37(6), 452 (2011).
A. V. Ivanov, O. V. Loseva, A. V. Gerasimenko, and V. I. Sergienko, Dokl. Phys. Chem. 426(1), 92 (2009).
A. V. Ivanov, V. I. Sergienko, A. V. Gerasimenko, et al., Russ. J. Coord. Chem. 36(5), 353 (2010).
A. V. Ivanov, S. A. Zinkin, V. I. Sergienko, et al., Russ. J. Inorg. Chem. 56, 409 (2011).
O. V. Loseva, T. A. Rodina, A. V. Ivanov, et al., Russ. J. Coord. Chem. 37(12), 897 (2011).
J. S. Casas, A. Sánchez, J. Bravo, et al., Inorg. Chim. Acta 158(1), 119 (1989).
A. V. Ivanov, A. V. Gerasimenko, A. A. Konzelko, et al., Inorg. Chim. Acta 359(12), 3855 (2006).
V. M. Byr’ko, Dithiocarbamates (Nauka, Moscow, 1984) [in Russian].
A. Pines, M. G. Gibby, and J. S. Waugh, J. Chem. Phys. 56(4), 1776 (1972).
P. G. Mennitt, M. P. Shatlock, V. J. Bartuska, and G. E. Maciel, J. Phys. Chem. 85(14), 2087 (1981).
J. J. H. Ackerman, T. V. Orr, V. J. Bartuska, and G. E. Maciel, J. Am. Chem. Soc. 101(2), 341 (1979).
W. H. Press, S. A. Teukolsky, W. T. Vetterling, and B. P. Flannery, Numerical Recipes in C (Cambridge Univ. Press, Cambridge, 1994).
P. Hodgkinson and L. Emsley, J. Chem. Phys. 107(13), 4808 (1997).
S. Wolfram, The Mathematica Book, 4th ed. (Wolfram Media/Cambridge Univ. Press, Cambridge, 1999).
SMART (Bruker AXS, Madison, Wis., 1998).
SAINT (Bruker AXS, Madison, Wis., 2003).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr. 64, 112 (2008).
N. Hannachi, K. Guidara, A. Bulou, and F. Hlel, Mater. Res. Bull. 45(11), 1754 (2010).
A. Bondi, J. Phys. Chem. 70(9), 3006 (1966).
N. W. Alcock, Adv. Inorg. Chem. Radiochem. 15(1), 1 (1972).
G. Exarchos, S. D. Robinson, and J. W. Steed, Polyhedron 20(24–25), 2951 (2001).
Encyclopedic Dictionary of Chemistry, Ed. by I. L. Knunyants (Sovetskaya Entsiklopediya, Moscow, 1983) [in Russian].
G. A. Razuvaev, G. V. Almazov, G. A. Domrachev, et al., Dokl. Akad. Nauk SSSR 294(1), 141 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.A. Rodina, A.V. Ivanov, O.V. Loseva, A.S. Zaeva, A.V. Gerasimenko, 2013, published in Zhurnal Neorganicheskoi Khimii, 2013, Vol. 58, No. 3, pp. 390–401.
Rights and permissions
About this article
Cite this article
Rodina, T.A., Ivanov, A.V., Loseva, O.V. et al. Chemisorption of the [AuCl4]− anion by cadmium cyclo-pentamethylenedithiocarbamate and the resulting bound-gold(III) species: The supramolecular structure and thermal behavior of the polynuclear complexes ([Au{S2CN(CH2)5}2]2[CdCl4]) n and ([Au{S2CN(CH2)5}Cl2]) n . Russ. J. Inorg. Chem. 58, 338–349 (2013). https://doi.org/10.1134/S0036023613030133
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023613030133