Abstract
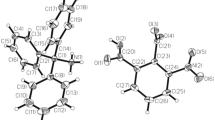
The reaction of thiamine chloride hydrochloride with a solution of palladium chloride in hydrochloric acid gave a protonated thiamine salt [HTA]2[PdCl4]Cl2 · 2H2O (I) (TA is 4-methyl-3-[(2′-methyl-4′-amino-3′,4′-dihydropyrimidinyl-5′)methyl]-5-(2-hydroxyethyl)thiazolium cation, C12H16N3O2S). The crystal structure of I was determined by X-ray diffraction. The crystals are triclinic: a = 11.459(8) Å, b = 12.239(8) Å, c = 6.910(1) Å, α = 103.24(3)°, β = 76.95(3)°, γ = 106.04(3)°, Z = 2, space group P \(\bar 1\). The structural units of I are doubly charged [HTA]2+ cations, [PdCl4]2− and Cl− anions, and crystallization water molecules combined by hydrogen bonds and electrostatic interactions. The planar thiazolium and pyrimidine rings are in the F conformation, Φt = 1.0°, Φp = −86.6°, and the dihedral angle between the planes is 85.5°. The torsion angles of the hydroxyethyl group are as follows: C(9)C(10)C(11)O(1), 175.6°; S(1)C(9)C(10)C(11), 33.2°; it is involved in the hydrogen bond with the free Cl− anion. The sulfur atom forms a short (3.052 Å) intermolecular S-Cl contact with the chlorine atom of the [PdCl4]2− anion, which forms supramolecular chains.
Similar content being viewed by others
References
S. V. Zabrodskaya, D. A. Oparin, and V. D. Makhaev, Vestsi Akad. Nauk Belarusi, Ser. Biyal. Navuk, 107 (1992).
Zh. V. Motylevich, S. V. Zabrodskaya, V. D. Makhaev, and D. A. Oparin, Proceedings of International Symposiium “Vitamins and Health of Polulations of Belarus and Neighboring Regions,” Grodno, 1995, p. 155.
C. V. Zabrodskaya, Zh. V. Motylevich, V. D. Makhaev, et al., RF Patent No. 2050361, 20.12.1995, Byul. Izobret., No. 35 (1995).
A. S. Antsyshkina, G. G. Sadikov, and V. D. Makhaev, Russ. J. Inorg. Chem. 53, 11703 (2008).
Hu Ning-Hai, K. Aoki, A. O. Adeyemo, and G. N. Williams, Inorg. Chim. Acta 325, 9 (2001).
R. E. Cramer, R. E. Kirkup, and M. J. J. Carrie, Inorg. Chem. 27, 123 (1988).
Hu. Ning-Hai, T. Tokuno, and K. Aoki, Inorg. Chim. Acta 295, 71 (1999).
W. Shin, J. Pletcher, G. Blank, and M. Sax, J. Am. Chem. Soc. 99, 3491 (1977).
F. Jordan, J. Am. Chem. Soc. 96, 3623 (1974).
J. Pletcher, M. Sax, G. Blank, and M. Wood, J. Am. Chem. Soc. 99, 1396 (1977).
A. S. Antsyshkina, G. G. Sadikov, and V. D. Makhaev, Russ. J. Inorg. Chem. 53, 1411 (2008).
G. M. Sheldrick, SHELXL-97, Program for the Refinement of Crystal Structures, Univ. of Göttingen, Germany, 1997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Antsyshkina, G.G. Sadikov, V.D. Makhaev, G.V. Shilov, 2012, published in Zhurnal Neorganicheskoi Khimii, 2012, Vol. 57, No. 7, pp. 1001–1005.
Rights and permissions
About this article
Cite this article
Antsyshkina, A.S., Sadikov, G.G., Makhaev, V.D. et al. Synthesis and crystal structure of the salt of the protonated thiamine cation with the palladium(II) tetrachloride anion [HTA]2[PdCl4]Cl2 · 2H2O. Russ. J. Inorg. Chem. 57, 927–931 (2012). https://doi.org/10.1134/S0036023612070042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023612070042