Abstract
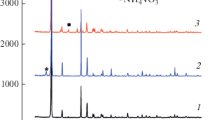
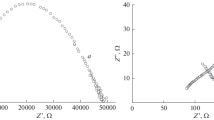
The phase and chemical compositions of the precipitates forming in the Sr(VO3)2-VOCl2-H2O system in the V4+/V5+ = 0.11–9 range at 80–90°C are reported. At pH 1–3 and V4+/V5+ = 0.25−9, the general formula of the precipitated compounds is Sr x V 4+ y V 5+12−y O31−δ·nH2)(0.37 ≤ x ≤ 1.0, 1.7 ≤ y ≤ 3.0, 0.95 ≤ δ ≤ 2.1). Polyvanadates containing the largest amount of vanadium(IV) are obtained at an initial V4+/V5+ ratio of 9 and pH 1.9. Precipitation from solutions at pH 3 takes place only in the presence of the VO2+ ion, and the highest precipitation rate is observed at V4+/V5+ = 0.11. The process is controlled by a second-order reaction on the polyvanadate surface. Under hydrothermal conditions at 180°C, Sr0.25V2O5·1.5H2O nanorods are obtained from solutions with a V4+/V5+ molar ratio of 0.1 at pH 3. The nanorods, 30–100 nm in diameter and up to 2–3 μm in length, have a layered structure with an interlayer spacing of 10.53 ± 0.08 Å.
Similar content being viewed by others
References
E. I. Burova, Uch. zap. Mosk. Gos. Univ. No. 6, 15 (1936).
A. A. Ivakin and V. L. Volkov, in Studies into the Chemistry and Technology of Mineral Salts and Oxides (Nauka, Moscow, 1965), p. 166 [in Russian].
A. A. Ivakin, I. G. Chufarova, V. G. Dobysh, and L. G. Borisyuk, Zh. Neorg. Khim. 30(4), 908 (1985).
V. L. Volkov and N. V. Podval’naya, Russ. J. Inorg. Chem. 45(12), 1912 (2000).
V. L. Volkov and N. V. Podval’naya, Russ. J. Inorg. Chem. 48(10), 1573 (2003).
N. V. Podval’naya and V. L. Volkov, Russ. J. Inorg. Chem. 51(3), 357 (2006).
N. V. Podval’naya and V. L. Volkov, Russ. J. Inorg. Chem. 52(9), 1468 (2007).
V. L. Volkov, N. V. Podval’naya, and E. G. Volkova, Russ. J. Inorg. Chem. 54(3), 350 (2009).
V. L. Volkov and G. S. Zakharova, Zh. Neorg. Khim. 33(4), 893 (1988).
I. G. Chufarova, A. A. Ivakin, N. I. Petunina, et al., Zh. Neorg. Khim. 24(4), 953 (1979).
I. G. Chufarova, A. A. Ivakin, N. I. Petunina, et al., Zh. Neorg. Khim. 26(10), 2826 (1981).
A. P. Nakhodnova and L. N. Kovgan, Zh. Neorg. Khim., No. 2, 387 (1975).
I. F. Poletaev, E. M. Gorbachevich, A. P. Lyudomirskaya, and E. M. Lyukmanova, Zh. Neorg. Khim. 32(5), 1060 (1987).
T. I. C. Rossotti and H. Rossotti, Acta Chem. Scand. 10(6), 957 (1956).
A. E. Nielsen, Acta Chem. Scand. 13(4), 784 (1959).
N. V. Podval’naya and V. L. Volkov, Russ. J. Inorg. Chem. 51(3), 357 (2006).
V. L. Volkov and N. V. Podval’naya, Russ. J. Inorg. Chem. 45(12), 1912 (2000).
N. V. Podval’naya and V. L. Volkov, Russ. J. Inorg. Chem. 52(9), 1468 (2007).
A. G. Swallow, F. R. Ahmed, and W. H. Barnes, Acta Crystallogr. 21(2), 397 (1966).
Author information
Authors and Affiliations
Additional information
Original Russian Text © N.V. Podval’naya, V.L. Volkov, 2011, published in Zhurnal Neorganicheskoi Khimii, 2011, Vol. 56, No. 12, pp. 2102–2107.
Rights and permissions
About this article
Cite this article
Podval’naya, N.V., Volkov, V.L. Composition and formation kinetics of strontium polyvanadates in vanadium(IV,V) solutions. Russ. J. Inorg. Chem. 56, 2013–2018 (2011). https://doi.org/10.1134/S0036023611120436
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023611120436