Abstract
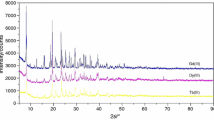
The thermal behavior of chitosanium dodecahydro-closo-dodecaborate, (C6O4H9NH3)2B12H12, was studied by thermal analysis, X-ray diffraction, and IR and X-ray photoelectron spectroscopy. As this compound is heated at a rate above 10–20 K/min, it ignites at a temperature of about 300°C. As the compound is heated to 1000°C at a rate below 10 K/min in an inert atmosphere, it yields a mixture of carbon and amorphous boron and/or boron carbides. The presence of a small amount of boron oxide in the product is explained by the formation of a partially oxidized hydroborate anion at the early stages of (C6O4H9NH3)2B12H12 decomposition via the interaction between oxygen of the chitosanium cation and the B12H 2−12 anion. Heating the initial compound in air at a rate below 10 K/min yields carbon and boron oxide as the main products. Molten boron oxide protects boron and/or boron carbides and boron nitride forming in small amounts in the particle bulk from oxidation.
Similar content being viewed by others
References
S. V. Ivanov, E. A. Malinina, K. A. Solntsev, and N. T. Kuznetsov, Koord. Khim. 18(4), 394 (1992).
L. I. Isaenko, K. G. Myakishev, I. S. Posnaya, and V. V. Volkov, Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim., No. 4, 73 (1982).
V. V. Volkov, K. G. Myakishev, and S. T. Dunaev, Izv. Akad. Nauk SSSR, Ser. Khim., No. 11, 2484 (1988).
V. V. Volkov, G. S. Yur’ev, L. Ya. Solomatina, et al., Izv. Akad. Nauk SSSR, No. 3, 503 (1990).
S. V. Ivanov, Candidate’s of Sciences Dissertation in Chemistry (IONKh RAN, Moscow, 1993) [in Russian].
V. I. Saldin, A. K. Tsvetnikov, L. N. Ignat’eva, and V. M. Buznik, Zh. Neorg. Khim. 49(11), 1908 (2004) [Russ. J. Inorg. Chem. 49 (11), (2004)].
V. I. Saldin, A. K. Tsvetnikov, L. N. Ignat’eva, et al., Zh. Neorg. Khim. 50(9), 1412 (2005) [Russ. J. Inorg. Chem. 50 (9), 1310 (2005)].
V. I. Saldin, L. N. Ignat’eva, and Yu. M. Nikolenko, Zh. Strukt. Khim. 47(1), 41 (2006).
E. A. Plisko, L. A. Nud’ga, and S. N. Danilov, Usp. Khim. 46(8), 1470 (1977).
A. K. Babko and I. V. Pyatnitskii, Quantitative Analysis (Vysshaya Shkola, Moscow, 1968) [in Russian].
E. L. Mueterthies, et al., Inorg. Chem. 84(3), 444 (1964).
W. H. Knoth, et al., J. Am. Chem. Soc. 84(6), 1056 (1962).
K. P. Callagan and M. F. Hawthorn, Recherche 8(6), 225 (1977).
N. T. Kuznetsov, Investigations into Inorganic Chemistry and Technology. Collected Works of the Institute of General and Inorganic Chemistry, Ed. by A. Yu. Tsivadze (Nauka, Moscow, 1988) [in Russian].
A. Clauss, R. Plass, H. P. Boehm, and U. Hoffman, Z. Anorg. Allg. Chem. 291(5–6), 205 (1957).
H. Bohem and W. Scholz, Justus Libbigs Ann. Chem. 691, 1 (1966).
W. Scholz and H. Bohem, Z. Anorg. Allg. Chem. 369, 113 (1969).
L. Y. Lim, et al., J. Biomed. Mater. Res. 48(2), 111 (1999).
K. Nakamoto, The Infrared Spectra of Inorganic and Coordination Compounds (Wiley, New York, 1963; Mir, Moscow, 1968).
H. Werhait, Proceedings of the 9th International Symposium on Boron, Borides, and Related Compounds (Duisburg, 1987), p. 142.
J. P. Riviere, M. Cahoreau, and Y. Pacaud, Thin Solid Films, No. 227, 44 (1993).
K. P. Burdina and O. V. Kravchenko, Proceedings of the 16th Mendeleev Congress on General and Applied Chemistry (Moscow, 1998), Section 2, p. 23 [in Russian].
M. Koh, H. Yumoto, Y. Higashi, and T. Nakajima, J. Fluor. Chem. 97, 239 (1999).
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.I. Saldin, L.N. Ignat’eva, Yu.M. Nikolenko, V.M. Buznik, Yu.M. Mikhailov, 2010, published in Zhurnal Neorganicheskoi Khimii, 2010, Vol. 55, No. 8, pp. 1296–1302.
Rights and permissions
About this article
Cite this article
Saldin, V.I., Ignat’eva, L.N., Nikolenko, Y.M. et al. Thermal conversions of chitosanium dodecahydro-closo-dodecaborate. Russ. J. Inorg. Chem. 55, 1221–1227 (2010). https://doi.org/10.1134/S0036023610080115
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023610080115