Abstract
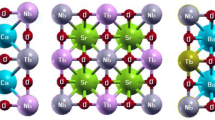
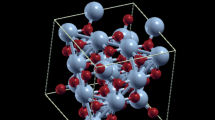
New perovskite oxide phases BaCe1 − x In x O3 − x/2 (x = 0.1–0.8) (space group Pbnm) have been synthesized. The unit cell volume of the resulting solid solutions monotonically decreases with an increase in the degree of substitution of indium for cerium due to the contraction of octahedra in perovskite blocks. The thermodynamic stability of the compound BaCe0.75In0.25O2.875 was studied by the solution calorimetry method, and barium cerates were shown to be thermodynamically stable with respect to binary oxides at room temperature. The structure of occupied and vacant states in BaCe1 − x In x O3 − x/2 was determined on the basis of X-ray emission, absorption, and photoelectron spectra, and the energy gap was estimated at ∼2 eV.
Similar content being viewed by others
References
K. D. Kreuer, Solid State Ionics 97, 1 (1997).
T. Takahashi and H. Iwahara, Rev. Chim. Miner. 17, 243 (1980).
K. Knight and N. Bonanos, Mater. Res. Bull. 30, 347 (1995).
K. D. Kreuer, Chem. Mater. 8, 610 (1996).
S. Steinvik, Y. Larring, and T. Norby, Solid State Ionics 143, 103 (2001).
G. C. Mather, F. M. Figueiredo, D. P. Fagg, et al., Solid State Ionics 158, 333 (2003).
A. V. Orlov, A. L. Vinokurov, O. A. Shlyakhtin, and Yu. D. Tretyakov, Mendeleev Commun. 14, 163 (2004).
A. P. Bobylev, M. N. Boubentsova, L. N. Komissarova, et al., Mendeleev Commun. 14, 146 (2004).
A. S. Vanetsev, V. K. Ivanov, N. N. Oleynikov, and Yu. D. Tretyakov, Mendeleev Commun. 14, 145 (2004).
N. Sammes, R. Philips, and A. Smirnova, J. Power Sources 134, 153 (2004).
A. Kruth, G. C. Mather, J. R. Jurado, and J. T. S. Irvine, Solid State Ionics 176, 703 (2005).
G. B. Zhang and D. M. Smyth, Solid State Ionics 82, 153 (1995).
K. Kunstler, H.-J. Lang, A. Maiwald, and G. Tomandl, Solid State Ionics 107, 221 (1998).
K. Takeuchi, S.-K. Loong, J. W. Richardson, et al., Solid State Ionics 138, 63 (2000).
J. A. Bearden, Dokl. Akad. Nauk SSSR 39, 1 (1967).
P. Berastegui, S. Hull, F. J. Garcia-Garcia, and S.-G. Eriksson, J. Solid State Chem. 164, 119 (2002).
T. Q. Ta, T. Tsuji, and Y. Yamamura, J. Alloys Comp. 408–412, 253 (2006).
C. Tenaileau, A. Pring, S. M. Moussa, et al., J. Solid State Chem. 178, 882 (2005).
S. Sasaki, C. T. Prewitt, and J. D. Bass, J. ACSCEE 43, 1668 (1987).
A. J. Jacobson, B. C. Tofield, and B. E. F. Fender, Acta Crystallogr., Sect. B: Struct. Sci. 28, 956 (1972).
M. Yoshimura, T. Nakamura, and T. Sata, Chem. Lett., 923 (1973).
R. D. Shannon, Acta Crystallogr., Sect. A: Found. Crystallogr. 32, 751 (1976).
H. Iwahara, T. Esaka, and H. Uchida, Solid State Ionics 3–4, 359 (1980).
J. Guan, S. E. Dorris, U. Balachandran, and M. Liu, Solid State Ionics 110, 303 (1998).
X. Qi and Y. S. Lin, Solid State Ionics 120, 85 (1999).
X. Qi and Y. S. Lin, Solid State Ionics 130, 149 (2000).
H. H. Huang, M. Ishigame, and S. Shin, Solid State Ionics 47, 251 (1991).
J. Valjakka, J. Utriainen, T. Åberg, and J. Tulkki, Phys. Rev. B: 32, 6892 (1985).
T. Higuchi, T. Tsukamoto, K. Kobayashi, et al., Phys. Rev. 65, 33201 (2002).
T. Higuchi, S. Yamaguchi, K. Kobayashi, et al., Jpn. J. Appl. Phys. 41, L938 (2002).
T. Higuchi, T. Tsukamoto, S. Yamaguchi, et al., Jpn. J. Appl. Phys. 42, L3526 (2003).
T. Higuchi, S. Yamaguchi, N. Sata, et al., Jpn. J. Appl. Phys. 42, L1265 (2003).
J. Valjakka, J. Utriainen, T. Åberg, and J. Tulkki, Phys. Rev. B: 32, 6892 (1985).
E. H. P. Cordfunke, A. S. Booij, and M. E. Huntelaar, J. Chem. Thermodyn. 30, 437 (1998).
L. V. Gurvich, Thermodynamic Properties of Individual Substances (Nauka, Moscow, 1982–1987), vols. 1–4.
N. I. Matskevich, G. Krabbes, and P. Berasteguie, Thermochim. Acta 397, 97 (2003).
N. I. Matskevich and Th. Wolf, Thermochim. Acta 421, 231 (2004).
Author information
Authors and Affiliations
Additional information
Original Russian Text © T.I. Chupakhina, N.I. Matskevich, G.V. Bazuev, N.A. Ovechkina, V.R. Galakhov, M. Raeckers, M. Neumann, 2010, published in Zhurnal Neorganicheskoi Khimii, 2010, Vol. 55, No. 7, pp. 1070–1077.
Rights and permissions
About this article
Cite this article
Chupakhina, T.I., Matskevich, N.I., Bazuev, G.V. et al. Synthesis, crystal and electronic structures, and thermodynamic characteristics of BaCe1 − x In x O3 − x/2 solid solutions. Russ. J. Inorg. Chem. 55, 1002–1009 (2010). https://doi.org/10.1134/S003602361007003X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602361007003X