Abstract
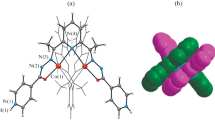
The synthesis of copper(II) binuclear complexes with acyldihydrazones of saturated carboxylic acids and 4-formyl-5-hydroxy-3-methyl-1-phenylpyrazoles in which the coordination polyhedra are connected by polymethylene chains of different length (two to five units) is described. The complexes were studied by chemical and thermal analysis, IR spectroscopy, and EPR. The molecular and crystal structure of the copper(II) complex with glutaric acid and 1-(4′-chlorophenyl)-4-formyl-5-hydroxy-3-methylpyrazole acyldihydrazone (H4L) described as [Cu2L·2Py] · Py · 4H2O was determined by X-ray diffraction. The crystals are orthorhombic: a = 24.789(7) Å, b = 39.319(9) Å, c = 4.6336(14) Å, space group Pnma, Z = 4. The number of symmetrically unrelated reflections is 4716, R = 0.0606, R w = 0.1307. The central atoms are separated by a chain of eight σ bonds and are located at a distance of 8.939 Å. The copper coordination polyhedron is a square. A specific feature of the crystal structure is the stacking interaction involving chelate rings and the pyrazole ring, resulting in stacks of molecular complexes. The EPR spectrum of a solution of the complex based on the acyldihydrazone of succinic acid and 4-formyl-5-hydroxy-3-methyl-1-phenylpyrazole recorded at room temperature exhibits seven HFS lines with an intensity ratio of 1: 2: 3: 4: 3: 2: 1 and a constant of 33.3 G as a result of exchange coupling of the unpaired electrons to the two equivalent copper nuclei. An increase in the length of the polymethylene chain to 3–5 units or introduction of the para-chlorine atom into the benzene ring hampers the exchange interactions, and the EPR spectrum shows a signal of four HFS lines with a constant of 55–70 G typical of monomeric copper(II) complexes.
Similar content being viewed by others
References
R. L. Carlin, Magnetochemistry (Spinger, Berlin, 1986; Mir, Moscow, 1989).
Yu. V. Rakitin and V. T. Kalinnikov, Modern Magnetochemistry (Nauka, St. Petersburg, 1994) [in Russian].
T. D. Smith and J. R. Pilbrow, Coord. Chem. Rev. 13(1), 173 (1974).
Yu. V. Yablokov, V. K. Voronkova, and L. V. Mosina, Paramagnetic Resonance of Exchange Clusters (Nauka, Moscow, 1988) [in Russian].
Yu. V. Rakitin, G. M. Larin, and V. V. Minin, Interpretation of EPR Spectra of Coordination Compounds (Nauka, Moscow, 1993) [in Russian].
G. M. Larin, B. B. Umarov, V. V. Minin, et al., Dokl. Akad. Nauk SSSR 303, 139 (1988).
G. M. Larin, V. V. Minin, and Yu. V. Rakitin, Neorg. Mater. 30(11), 1424 (1994).
G. M. Larin, V. F. Shul’gin, E. A. Sarnit, and Yu. V. Rakitin, Koord. Khim. 25(5), 356 (1999) [Russ. J. Coord. Chem. 25 (5), 333 (1999)].
G. M. Larin, V. F. Shul’gin, E. A. Sarnit, and Yu. V. Rakitin, Izv. Akad. Nauk, Ser. Khim., No. 5, 777 (2001).
V. F. Shul’gin, A. N. Gusev, V. Ya. Zub, and G. M. Larin, Izv. Akad. Nauk, Ser. Khim., No. 12, 2107 (2002).
V. F. Shul’gin, A. N. Gusev, V. Ya. Zub, and G. M. Larin, Izv. Akad. Nauk, Ser. Khim., No. 6, 1230 (2003).
V. F. Shul’gin, A. N. Gusev, V. Ya. Zub, and G. M. Larin, Izv. Akad. Nauk, Ser. Khim., No. 8, 1752 (2005).
V. F. Shul’gin, A. N. Gusev, A. N. Chernega, and G. M. Larin, Izv. Akad. Nauk, Ser. Khim., No. 2, 229 (2007).
B. A. Porai-Koshits and I. Ya. Kvitko, Zh. Obshch. Khim. 34(9), 2995 (1964).
G. M. Sheldrick, SHELX97, Program for the Solution of Crystal Structures, Göttingen Univ., Göttingen (Germany), 1997.
I. Ya. Kvitko and B. A. Porai-Koshits, Zh. Obshch. Khim. 34(9), 3005 (1964).
I. Ya. Kvitko, L. B. Alam, M. N. Bobrovnikov, et al., Zh. Obshch. Khim. 64(4), 657 (1994).
F. H. Allen, O. Kennard, D. G. Watson, et al., J. Chem. Soc., Perkin Trans. 2, S1 (1987).
Author information
Authors and Affiliations
Additional information
Original Russian Text © V.F. Shul’gin, A.I. Obukh, E.B. Rusanov, V.Ya. Zub, V.V. Minin, 2009, published in Zhurnal Neorganicheskoi Khimii, 2009, Vol. 54, No. 8, pp. 1288–1295.
Rights and permissions
About this article
Cite this article
Shul’gin, V.F., Obukh, A.I., Rusanov, E.B. et al. Binuclear copper(II) complexes with acyldihydrazones of 4-formyl-5-hydroxy-3-methyl-1-phenylpyrazoles. Russ. J. Inorg. Chem. 54, 1223–1229 (2009). https://doi.org/10.1134/S0036023609080099
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023609080099