Abstract
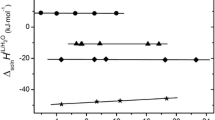
The heats of solution of tetrahexylammonium and tetraheptylammonium bromides in water were measured at 318.15 and 328.15 K. The standard enthalpies and specific heats of solution and the temperature changes in the free energy and entropy of solution were calculated. A comparison of the thermodynamic properties of solutions for the homologous series of tetraalkylammonium salts demonstrated that the enthalpic, entropic, and specific heat characteristics of solution are positive and increase almost linearly up to tetrapentylammonium bromide. On passing to larger homologues, these parameters decrease, suggesting that the hydrophobic hydration effect is substantially weaker in solutions of tetraalkylammonium salts with larger cations.
Similar content being viewed by others
References
W. Kauzmann, Adv. Prot. Chem. 14, 1 (1959).
Yu. M. Kessler and A. L. Zaitsev, Solvophobic Effects: Theory, Experiment, and Practice (Khimiya, Leningrad, 1989) [in Russian].
L. R. Pratt, Ann. Rev. Phys. Chem. 52, 1 (2002).
A. K. Lyashchenko, in Theoretical and Applied Inorganic Chemestry (Nauka, Moscow, 1999), p. 60 [in Russian].
K. Lum, D. Chandler, and J. D. Weeks, J. Phys. Chem. B 103, 4570 (1999).
D. M. Huang, P. L. Geissler, and D. Chandler, J. Phys. Chem. B 105, 6704 (2001).
D. M. Huang and D. Chandler, J. Phys. Chem. B 106, 2047 (2002).
D. Chandler, Nature 445, 831 (2007).
J. Z. Tuner and A. K. Soper, J. Chem. Phys. 101, 6116 (1994).
N. G. Polydorou, J. D. Wicks, and J. Z. Tuner, J. Chem. Phys. 107(1), 197 (1997).
B. Madan and K. Sharp, Biophys. Chem. 78, 33 (1999).
A. V. Kustov and V. P. Korolev, Zh. Fiz. Khim. 80(1), 64 (2006) [Russ. J. Phys. Chem. 80 (1), 56 (2006)].
O. Ya. Samoilov, Yu. V. Ergin, and L. I. Kostrova, Zh. Strukt. Khim. 17(4), 646 (1976).
H. L. Friedman and C. V. Krishnan, J. Phys. Chem. 75, 3606 (1971).
H. Nakayama, H. Kuwata, N. Yamamoto, et al., Bull. Chem. Soc. Jpn. 62, 985 (1989).
A. V. Kustov, A. A. Emel’yanov, A. F. Syshchenko, et al., Zh. Fiz. Khim. 80(9), 1724 (2006) [Russ. J. Phys. Chem. 80 (9), 1532 (2006)].
S. N. Solov’ev, N. M. Privalova, and A. F. Vorob’ev, Available from VINITI, No. 2101-76 (1976).
N. G. Manin, T. N. Lapshina, and V. P. Korolev, Zh. Fiz. Khim. 71(8), 1351 (1997) [Russ. J. Phys. Chem. 71 (8), 1207 (1997)].
N. G. Manin, I. B. Kurbanov, and V. P. Korolev, Zh. Fiz. Khim. 73(3), 475 (1999) [Russ. J. Phys. Chem. 73 (3), 400 (1999)].
M. Castagnolo, A. Sacco, and A. de Ciglio, J. Chem. Soc., Faraday Trans. 80, 2669 (1984).
M. H. Abraham and Y. Marcus, J. Chem. Soc., Faraday Trans. 1 82, 3255 (1986).
Y. Nagano, M. Sakiyama, T. Fujiwara, and Y. Kondo, J. Phys. Chem. 92, 5823 (1988).
Y. Nagano, H. Mizuno, M. Sakiyama, et al., J. Phys. Chem. 95, 2536 (1991).
A. M. Kolker and G. A. Krestov, Contemporary Issues of Solution Chemistry, Ed. by B. D. Berezin (Nauka, Moscow, 1986) [in Russian].
H. S. Frank and M. W. Evans, J. Chem. Phys. 13(507) (1945).
S. Cabani, P. Gianni, V. Mollica, and L. Lepori, J. Solution Chem 10, 563 (1981).
A. Ben-Naim and Y. Marcus, J. Chem. Phys. 81, 2016 (1984).
F. Franks, Water: A Comprehensive Treatise. Ch. 1 (Plenum, New York, 1975), Vol. 4, p. 1.
K. R. Gallaher and K. A. Sharp, J. Am. Chem. Soc. 125, 9853 (2003).
V. S. Dynyashev, L. V. Dynyashev, and A. K. Lyaschenko, Abstr. XVI International Conf. on Chem. Thermodyn. Susdal, 2007, p. 5/S–617.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Kustov, 2009, published in Zhurnal Neorganicheskoi Khimii, 2009, Vol. 54, No. 2, pp. 368–373.
Rights and permissions
About this article
Cite this article
Kustov, A.V. Thermodynamic properties of aqueous solutions of tetraalkylammonium salts: Dependence of the hydrophobic hydration effect on the cation size. Russ. J. Inorg. Chem. 54, 323–328 (2009). https://doi.org/10.1134/S0036023609020284
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023609020284