Abstract
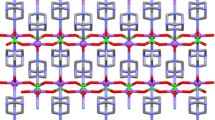
Features of proton-for-lithium ion substitution in monoclinic and pseudocubic lithium titanate are studied by X-ray diffraction, NMR spectroscopy, and Raman spectroscopy. Proton incorporation into the lithium titanate structure decreases the parameter a c of the pseudocubic unit cell from 8.28 Å in Li2TiO3 to =8.15 Å in H2TiO3. Metatitanic acid, like hydrous titania, has weak acid properties, but unlike titania, it sorbs hydrolyzable multicharged cations from aqueous solutions.
Similar content being viewed by others
References
V. M. Gun’ko, Teor. Eksp. Khim., 36(1), 1 (2000).
M. A. Henderson, Surf. Sci. Rep. 46, 1 (2002).
I. A. Udalova, V.I. Ivanenko, and V.T. Kalinnikov, Zh. Neorg. Khim. 47(6), 1033 (2002) [Russ. J. Inorg. Chem. 47 (6), 930 (2002)]
T. Sugimoto, X. Zhou, and A. Muramatsu, J. Colloid Interface Sci. 252, 339 (2002).
T. Sugimoto and X. Zhou, J. Colloid Interface Sci. 252, 347 (2002).
U. Diebold, Surf. Sci. Rep. 48, 53 (2003).
H. Izawa and S. Hikawa, Solid State Chem. 69, 336 (1987).
O. Kenta, M. Yoshitaka, and K. Shunsaka, Jpn. Kokai Tokkio Koho (1989).
S. Uchida, Y. Yamamoto, Y. Fujishiro, et al., J. Phys. Soc., Faraday Trans 93, 3229 (1997).
M. Yanagisava, S. Uchida, S. Yin, and T. Sato, Chem. Mater. 13, 174 (2001).
G. S. Zakharova, V. L. Volkov, A. L. Ivanovskii, and V. V. Ivanovskaya, Nanotubes and Related Nanostructures of Metal Oxides (Ural. Otd. Ross. Akad. Nauk, Yekaterinburg, 2005) [in Russian].
T. A. Denisova, M. V. Kuznetsov, V. L. Kozhevnikov, et al., Proceedings of the International Symposium OMA-II, Sochi, 2001 (Rostov, 2001), p. 9.
V. M. Zainullina, V. P. Zhukov, T. A. Denisova, and L. G. Maksimova, Zh. Strukt. Khim. 44(2), 210 (2003).
E. V. Polyakov, Reactions of Colloidal Ion Species of Microcomponents and Radionuclides in Aqueous Solutions (Ural. Otd. Ross. Akad. Nauk, Yekaterinburg, 2003) [in Russian].
M. Castellanos and A. R. West, J. Mater. Sci. 14, 450 (1979).
D. C. Johnston, J. Low Temp. Phys. 25(12), 145 (1976).
Yu. V. Shchapova, E. I. Yur’eva, M. V. Ryzhkov, et al., Quantum-Chemical Calculations in Mineralogy: Simulation of the Electronic Structure and Mössbauer Spectral Parameters (IMin UrO RAN, Miass, 2000) [in Russian].
R. N. Pletnev, A. A. Ivakin, D. G. Kleshchev, et al., Hydrated Oxides of the Group IV and V Elements (Nauka, Moscow, 1986) [in Russian].
A. G. Lundin and E. I. Fedin, Nuclear Magnetic Resonance (Nauka, Novosibirsk, 1980) [in Russian].
V. M. Mastikhin and K. I. Zamaraev, Heterogeneous Catalysis as Probed by Solid-State High-Resolution NMR (Inst. of Catalysis, Novosibirsk, 1990).
N. V. Porotnikov, N. G. Chaban, and K. I. Petrov, Zh. Neorg. Khim. 28(10), 2466 (1983).
L. V. Golubeeva, N. V. Porotnikov, O. I. Kondratov, and K. I. Petrov, Zh. Neorg. Khim. 35(7), 1804 (1990).
V. G. Soldatov, in Ion Exchange (Nauka, Moscow), p. 111 [in Russian].
S. I. Pechenyuk, Izv. Akad. Nauk, Ser. Khim., No. 2, 229 (1999).
Author information
Authors and Affiliations
Additional information
Original Russian Text © T.A. Denisova, L.G. Maksimova, E.V. Polyakov, N.A. Zhuravlev, S.A. Kovyazina, O.N. Leonidova, D.F. Khabibulin, E.I. Yur’eva, 2006, published in Zhurnal Neorganicheskoi Khimii, 2006, Vol. 51, No. 5, pp. 757–766.
Rights and permissions
About this article
Cite this article
Denisova, T.A., Maksimova, L.G., Polyakov, E.V. et al. Metatitanic acid: Synthesis and properties. Russ. J. Inorg. Chem. 51, 691–699 (2006). https://doi.org/10.1134/S0036023606050019
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0036023606050019