Abstract
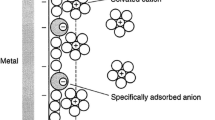
Equilibrium state of a two-phase system (metal-solution) is conventionally considered (see e.g. [1–3]) as a reversible process of the charge transfer by a metal cation through the interface boundary (this concept is applied to both first-kind electrodes and second-kind electrodes reversible in the anion of the metal salt, as well as redox electrodes, which may be reduced to the first-kind electrodes using the Luther rule). However, it is actually an electron that is the main charged component in all these exchanges. The exchange processes cannot be directly measured at the molecular level, while the electron current in the external circuit of the galvanic cell can be measured quite accurately. The current and potential (the potential difference between the studied and auxiliary electrodes) are the main characteristics of the electrode processes. Theoretical analysis of these phenomena by an example of electrolysis is the object of the present study.
Similar content being viewed by others
References
Damaskin, B.B., Petrii, O.A., and Tsirlina, G.A., Elektrokhimiya (Electrochemistry), Moscow: Khimiya, 2001.
Frumkin, A.N., Potentsialy nulevogo zaryada (Zero Point Potentials), Moscow: Nauka, 1979.
Fetter, K., Elektrochemische kinetik, Berlin: Springer, 1961.
Theory of the Inhomogeneous Electron Gas, Lundquist, S. and March, N.H., Eds., New York: Plenum, 1983.
Salem, R.R., Nachala teoreticheskoi elektrokhimii (Bases of Theoretical Electrochemistry), Moscow: KomKniga, 2005.
Kohn, W. and Sham, L.J., Phys. Rev., 1965, vol. 140, no. 56, p. 2166.
Rice, O.K., Phys. Rev., 1928, vol. 31, p. 1051.
Hart, E.J. and Anbar, M., The Hydrated Electron, New York: Wiley.
Kuz’min, M.G., In Fizicheskaya khimiya. Sovremennye problemy (Physical Chemistry. Modern Problems), Moscow: Khimiya, 1988, p. 31.
Salem, R.R., Teoriya dvoinogo sloya (Theory of Double Layer), Moscow: Fizmatlit, 2003.
Pikaev, A.K., Sol’vatirovannyi elektron v radiatsionnoi khimii (Solvated Electron in Radiation Chemistry), Moscow: Nauka, 1969.
Martynov, G.A. and Salem, R.R., Zashch. Met., 1985, vol. 21, no. 2, p. 221.
Blakemore, J.S., Solid State Physics, Cambridge: Cambridge University Press.
Gamov, G., Zh. Phyz., 1928, vol. 51, p. 204.
Krishtalik, L.I., Elektrodnye reaktsii, Melkhanizm elementarnogo akta (Electrode Reactions. Elementary Act Mechanism), Moscow: Nauka, 1982.
Benderskii, V.A. and Ovchinnikov, A.A., In Fizicheskaya khimiya. Sovremennye problemy (Physical Chemistry. Modern Problems), Moscow: Khimiya, 1980, p. 203.
Salem, R.R., Theodor Grotthuss Electrochemistry Conference, Book of Abstracts, Bilnius, 2005, p. 79.
Salem, R.R., Zashch. Met., 2006, vol. 42, no. 1, p. 67.
Salem, R.R., Elementy termodinamiki neobratimykh protsessov (Elements of Thermodynamics of Irreversible Processes), Moscow: VKhK RAN, 1994.
Heyrovsky, J. and Kuta, J., Zaklady Polarografie. Naklada telstvi, Cheskoslovenske Akademie Ved, Praha, 1962.
Khomutov, N.E., Itogi Nauki. Elektrokhimiya, 1967.
Spravochnik po elektrokhimii (Handbook on Electrochemistry), Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Malenkov, G.G., in Fizicheskaya khimiya. Sovremennye problemy (Physical Chemistry. Modern Problems), Moscow: Khimiya, 1984, p. 77.
Additional information
Original Russian Text © R.R. Salem, 2006, published in Zashchita Metallov, 2006, Vol. 42, No. 6, pp. 574–583.