Abstract
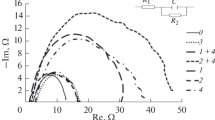
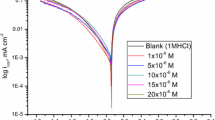
The corrosion and electrochemical behavior of carbon steels in standard 0.5% chloride electrolytes acidified with CH3COOH and saturated with H2S was studied. The possibility of protecting steels with quaternary ammonium salts (QASs) obtained by reactions of secondary and tertiary amines with benzyl chloride was investigated. It was found that the protective properties of QASs depend on their chemical structures and can be expressed, to a first approximation, in terms of the Linear Gibbs Energy Relation with the use of the hydrophobic f constants and Taft σ*-constants of the substituents. The presence of hydrophobic and electron-accepting substituents was found to enhance the protective properties of the inhibitors.
Similar content being viewed by others
References
Rozenfel’d, I.L., Ingibitory korrozii (Corrosion Inhibitors), Moscow: Khimiya, 1977, p. 350.
Saakyan, L.S. and Efremov, A.P., Zashchita neftegazopromyslovogo oborudovaniya ot korrozii (Protection of Oil-and Gas-Field Equipment from Corrosion), Moscow: Nedra, 1982.
Gumerova, A.G., Khudyakova, L.P., and Pirogov, A.G., Monit. Bezop. Truboprovodn. Sist., 2004, no. 1, p. 3.
Antropov, L.I. and Panasenko, V.F., Itogi Nauki Tekh., Ser.: Korroz. Zashch. Korroz, Moscow: VINITI, 1975, vol. 4, p. 46.
Rozenfel’d, I.L., Frolova, L.V., and Brusnikina, V.M., Sov. Sci. Rev. Sect. B: Chem. Rev., Amsterdam: OPA, 1987, vol. 8, p. 115.
Kuznetsov, Yu.I. and Frolova, L.V., Korroz. Mater. Zashch., 2004, no. 8, p. 11.
Petrov, N.A., Yur’ev, V.M., Enikeev, E.Kh., et al., Sintez i podbor ingibitorov korrozii dlya zashchity oborudovaniya i truboprovodov v H 2 S sredakh. Obzor informatsii (Synthesis and Selection of Corrosion Inhibitors for Protection of Equipment and Pipelines in H2S Media. A Survey of Published Materials), Moscow: Eridan-Ekspo, 1995.
Kuznetsov, Yu.I. and Vagapov, R.K., Zashch. Met., 2000, vol. 36, no. 5, pp. 520–524.
Hansch, C. and Leo, A., Substituent Constants for Correlation Analysis in Chemistry and Biology, New York: Wiley, 1979.
Dupin, P., Viliria-Vera, D.A., de Savignac, A., et al., Proc. 5th Eur. Symp. on Corrosion Inhibitors, Ferrara, 1980, vol. 1, p. 301.
Kuznetsov, Yu.I., Organic Inhibitors of Corrosion of Metals, New York: Plenum, 1996.
Zhdanov, Yu.A. and Minkin, V.I., Korrelyatsionnyi analiz v organicheskoi khimii (Correlation Analysis in Organic Chemistry), Rostov-on-Don: Rostov. Univ., 1966.
Grigor’ev, V.P. and Ekilik, V.V., Khimicheskaya struktura i zashchitnoe deistvie ingibitorov korrozii (Corrosion Inhibitors: Chemical Structure and Protective Effect), Rostov-on-Don: Rostov. Univ., 1978.
Roeloff, F. and Rekker, The Hydrophobic Fragmental Constants, Amsterdam, 1977, p. 48.
Author information
Authors and Affiliations
Additional information
Original Russian Text © Yu.I. Kuznetsov, L.V. Frolova, E.V. Tomina, 2006, published in Zashchita Metallov, 2006, Vol. 42, No. 3, pp. 233–238.
Rights and permissions
About this article
Cite this article
Kuznetsov, Y.I., Frolova, L.V. & Tomina, E.V. On inhibition of hydrogen-sulfide corrosion of steels with quaternary ammonium salts. Prot Met 42, 215–220 (2006). https://doi.org/10.1134/S0033173206030027
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0033173206030027