Abstract—
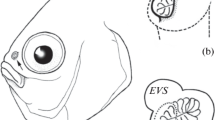
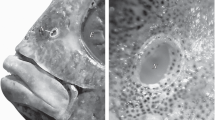
The macromorphology of olfactory organ was studied in moon wrasse Thalassoma lunare and sixbar wrasse T. hardwicke. There are two nostrils in the olfactory organ. Thin walls of the anterior tubular nostril can easily close, the posterior nostril contains a valve. The olfactory rosette is absent and is replaced by the olfactory disk and vertical membrane, morphology structures of the olfactory organ previously unknown in fish. The disk is located on the rostral bottom part of olfactory cavity. Low crest-folds cover the disk surface that are probably the vestigial structures of primary olfactory folds. The expressiveness of crest-folds, disk thickness and shape are different in the moon wrasse and sixbar wrasse. In both species only one large ventilation lacrimal nasal sac is present adjacent below the olfactory cavity with the entrance at the cavity bottom caudal from the disk. A scheme of ventilation of the olfactory cavity is introduced. The possibility of receiving the olfactory information is discussed for wrasses staying in the ground at the time of avoidance of danger.







Similar content being viewed by others
REFERENCES
Aiello, B.R., Westneat, M.W., and Hale, M.E., Mechanosensation is evolutionarily tuned to locomotor mechanics, Proc. Natl. Acad. Sci. U.S.A., 2017, vol. 114, no. 17, pp. 4459–4464. https://doi.org/10.1073/pnas.1616839114
Atema, J., Kingsford, M.J., and Gerlach, G., Larval reef fish could use odour for detection, retention and orientation to reefs, Mar. Ecol.: Prog. Ser., 2002, vol. 241, pp. 151–160. https://doi.org/10.3354/meps241151
Barry, K.L. and Hawryshyn, C.W., Spectral sensitivity of the Hawaiian saddle wrasse, Thalassoma duperrey, and implications for visually mediated behavior on coral reefs, Environ. Biol. Fish., 1999, vol. 56, pp. 429–442. https://doi.org/10.1023/A:1007556112449
Belanger, R.M., Smith, C.M., Corkum, L.D., and Zielinski, B.S., Morphology and histochemistry of the peripheral olfactory organ in the round goby, Neogobius melanostomus (Teleostei: Gobiidae), J. Morphol., 2003, vol. 257, pp. 62–71. https://doi.org/10.1002/jmor.10106
Bellwood, D.R., Wainwright, P.C., Fulton, C.J., and Hoey, A.S., Functional versatility supports coral reef biodiversity, Proc. R. Soc. B, 2006, vol. 273, pp. 101–107. https://doi.org/10.1098/rspb.2005.3276
Biswas, S., Datta, N.C., Sarkar, S.K., and De, S.K., Anatomical variation in the olfactory apparatus of marine teleosts, J. Res. Biol., 2013, vol. 3, no. 1, pp. 742–748.
Boyle, K.S. and Cox, T.E., Courtship and spawning sounds in bird wrasse Gomphosus varius and saddle wrasse Thalassoma duperrey, J. Fish Biol., 2009, vol. 75, pp. 2670–2681. https://doi.org/10.1111/j.1095-8649.2009.02459.x
Braun, C., Michiels, N.K., Siebeck, U.E., and Sprenger, D., Signaling function of long wavelength colors during agonistic male–male interactions in the wrasse Coris julis, Mar. Ecol.: Prog. Ser., 2014, vol. 504, pp. 277–286. https://doi.org/10.3354/meps10760
Brown, C., Tool use in fishes, Fish Fish., 2012, vol. 13, pp. 105–115. https://doi.org/10.1111/j.1467-2979.2011.00451.x
Burne, R.H., The anatomy of the olfactory organ of teleostean fishes, Proc. Zool. Soc. London, 1909, vol. 2, pp. 610–663.
Chateau, O. and Wantiez, L., Site fidelity and activity patterns of a humphead wrasse, Cheilinus undulatus (Labridae), as determined by acoustic telemetry, Environ. Biol. Fish., 2007, vol. 80, pp. 503–508. https://doi.org/10.1007/s10641-006-9149-6
Cheney, K.L., Bshary, R., and Grutter, A.S., Cleaner fish cause predators to reduce aggression toward bystanders at cleaning stations, Behav. Ecol., 2008, vol. 19, pp. 1063–1067. https://doi.org/10.1093/beheco/arn067
Colefax, A.P., Haywood, M.D.E., and Tibbetts, I.R., Effect of angling intensity on feeding behavior and community structure of subtropical reef‑associated fishes, Mar. Biol., 2016, vol. 163, pp. 1–14. https://doi.org/10.1007/s00227-016-2857-3
Colin, P.L., Aggregation and spawning of the humphead wrasse Cheilinus undulatus (Pisces: Labridae): general aspects of spawning behavior, J. Fish Biol., 2010, vol. 76, pp. 987–1007. https://doi.org/10.1111/j.1095-8649.2010.02553.x
Collar, D.C., Wainwright, P.C., and Alfaro, M., Integrated diversification of locomotion and feeding in labrid fishes, Biol. Lett., 2008, vol. 4, pp. 84–86. https://doi.org/10.1098/rsbl.2007.0509
Coppock, A.G., Gardiner, N.M., and Jones, G.P., Olfactory discrimination in juvenile coral reef fishes: response to conspecifics and corals, J. Exp. Mar. Biol. Ecol., 2013, vol. 443, pp. 21–26. https://doi.org/10.1016/J.JEMBE.2013.02.026
Coppock, A.G., Gardiner, N.G., and Jones, G.P., Olfactory responses of coral-reef fishes to coral degradation and crown-of-thorns (Acanthaster planci), Mar. Freshwater Res., 2016, vol. 67, pp. 605–611. https://doi.org/10.1071/MF14424
Cowman, P.F., Bellwood, D.R., and van Herwerden, L., Dating the evolutionary origins of the wrasses (Labridae) and the rise of trophic novelty on coral reefs, Mol. Phylogenet. Evol., 2009, vol. 52, pp. 621–631. https://doi.org/10.1016/j.ympev.2009.05.015
Coyer, J., Use of a rock as an anvil for breaking scallops by the yellowhead wrasse, Halichoeres garnoti (Labridae), Bull. Mar. Sci., 1995, vol. 57, pp. 548–549.
DeLoach, N., Reef Fish Behavior: Florida, Caribbean, Bahamas, Jacksonville, FL: New World, 1999.
Dixson, D.L., Jones, G.P., Munday, P.L., et al., Coral reef fish smell leaves to find island homes, Proc. R. Soc. B, 2008, vol. 275, pp. 2831–2839. https://doi.org/10.1098/rspb.2008.0876
Døving, K.B., Functional properties of the fish olfactory system, in Progress in Sensory Physiology, Berlin: Springer-Verlag, 1986, vol. 6, pp. 39–104.
Døving, K.B., Dubois-Dauphin, M., Holley, A., and Jourdan, F., Functional anatomy of the olfactory organ of fish and the ciliary mechanism of water transport, Acta Zool., 1977, vol. 58, pp. 245–255.
Døving K.B., Stabell, O.B., Östlund-Nilsson, S., and Fisher, R., Site fidelity and homing in tropical coral reef cardinalfish: are they using olfactory cues? Chem. Sens., 2006, vol. 31, pp. 265–272. https://doi.org/10.1093/chemse/bjj028
Dunn, R.P., Tool use by a temperate wrasse, California sheephead Semicossyphus pulcher, J. Fish Biol., 2016, vol. 88, no. 2, pp. 805–810. https://doi.org/10.1111/jfb.12856
Eckes, M.J., Siebeck, U.E., Dove, S., and Grutter, A.S., Ultraviolet sunscreens in reef fish mucus, Mar. Ecol.: Prog. Ser., 2008, vol. 353, pp. 203–211. https://doi.org/10.3354/meps07210
Eschmeyer’s Catalog of Fishes: Genera, Species, References, Fricke, R., Eschmeyer, W.N., van der Laan, R., Eds., 2018. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp. Accessed December, 2020.
Ferry-Graham, L.A., Wainwright, P.C., Westneat, M.W., and Bellwood, D.R., Mechanisms of benthic prey capture in wrasses (Labridae), Mar. Biol., 2002, vol. 141, pp. 819–830. https://doi.org/https://doi.org/10.1007/s00227-002-0882-x
Francini-Filho, R.B. and Sazima, I., A comparative study of cleaning activity of two reef fishes at Fernando de Noronha Archipelago, tropical West Atlantic, Environ. Biol. Fish., 2008, vol. 83, pp. 213–220. https://doi.org/10.1007/s10641-007-9322-6
Fulton, C.J. and Bellwood, D.R., Patterns of foraging in labrid fishes, Mar. Ecol.: Prog. Ser., 2002, vol. 226, pp. 135–142. http://www.int-res.com/abstracts/meps/v226
Gerlach, G., Atema, J., Kingsford, M.J., et al., Smelling home can prevent dispersal of reef fish larvae, Proc. Natl. Acad. Sci. U.S.A., 2007, vol. 104, pp. 858–863. https://doi.org/10.1073_pnas.0606777104
Goemans, B., Thalassoma hardwicke (Bennett, 1828), Version 04/2019, 2012. http://www.saltcorner.com/AquariumLibrary/browsespecies.php?CritterID=1964.
Gooding, K.B., The olfactory organ of the skipjack, Katsuwonus pelamis, FAO Fish Rep., 1963, vol. 6, pp. 1621–1631.
Grutter, A.S., Ontogenetic variation in the diet of the cleaner fish Labroides dimidiatus and its ecological consequences, Mar. Ecol.: Prog. Ser., 2000, vol. 197, pp. 241–246. https://doi.org/10.3354/meps197241
Grutter, A.S., Rumney, J.G., Sinclair-Taylor, T., et al., Fish mucous cocoons: the ‘mosquito nets’ of the sea, Biol. Lett., 2011, vol. 7, pp. 292–294. https://doi.org/10.1098/rsbl.2010.0916
Helfman, G.S., Collette, B.B., and Facey, D.E., The Diversity of Fishes, Oxford: Blackwell, 1997.
Holl, A. and Meinel, W., Das Geruchsorgan des Tiefseefisches Aphanopus carho (Percomorphi, Trichiuridae), Helgol. Wiss. Meeresunters., 1968, vol. 18, pp. 404–423. https://doi.org/10.1007/BF01611678
Holmes, T.H., Wilson, S.K., Vanderklift, M., et al., The role of Thalassoma lunare as a predator of juvenile fish on a sub-tropical coral reef, Coral Reefs, 2012, vol. 31, pp. 1113–1123. https://doi.org/10.1007/s00338-012-0934-8
Holstein, D.M., Paris, C.B., and Mumby, P.J., Consistency and inconsistency in multispecies population network dynamics of coral reef ecosystems, Mar. Ecol.: Prog. Ser., 2014, vol. 499, pp. 1–18. https://doi.org/10.3354/meps10647
Hourigan, T.F., Nakamura, N., Nagahama, Y., et al., Histology, ultrastructure, and in vitro steroidogenesis of the testes of two male phenotypes of the protogynous fish, Thalassoma duperrey (Labridae), Gen. Comp. Endocrinol., 1991, vol. 83, pp. 193–217. https://doi.org/10.1016/0016-6480(91)90023-y
Jones, A., Brown, C., and Gardener, S., Tool use in the spotted tuskfish, Choerodon schoenleinii? Coral Reefs, 2011, vol. 30, p. 865. https://doi.org/10.1007/s00338-011-0790-y
Jones, K.M.M., Home range areas and activity centers in six species of Caribbean wrasses (Labridae), J. Fish Biol., 2005, vol. 66, pp. 150–166. https://doi.org/10.1111/j.1095-8649.2004.00589.x
Jones, K.M.M., Distribution of behaviours and species interactions within home range contours in five Caribbean reef fish species (family Labridae), Environ. Biol. Fish., 2007, vol. 80, pp. 35–49. https://doi.org/10.1007/s10641-006-9104-6
Jones, M.C., Grutter, A.S., and Cribb, T.H., Cleaner fish become hosts: a novel form of parasite transmission, Coral Reefs, 2004, vol. 23, pp. 521–529. https://doi.org/10.1007/s00338-004-0411-0
Kapoor, A.S. and Ojha, P.P., Studies on ventilation of the olfactory chambers of fishes with a critical reevaluation of the role of the accessory nasal sacs, Arch. Biol., 1972, vol. 83, pp. 167–178.
Kapoor, A.S. and Ojha, P.P., Functional anatomy of the nose and accessory nasal sacs in the teleost Channa punctatus Bloch., Acta Anat. (Basel), 1973, vol. 84, no. 1, pp. 84–96.
Kasumyan, A.O., The olfactory system in fish: structure, function, and role in behavior, J. Ichthyol., 2004, vol. 44, no. 2, pp. S180–S223.
Kasumyan, A.O., Pashchenko, N.I., and Oan’, L.T.K., Morphology of the olfactory organ in the climbing perch (Anabas testudineus Anabantidae, Perciformes), Zool. Zh., 2021, vol. 100, no. 1, pp. 40–56.
Kazancıoğlu, E., Near, T.J., Hanel, R., and Wainwright, P.C., Influence of sexual selection and feeding functional morphology on diversification rate of parrotfishes (Scaridae), Proc. R. Soc. B, 2009, vol. 276, pp. 3439–3446. https://doi.org/10.1098/rspb.2009.0876
Khaparde, K.P., Baile, V.V., Masram, S.C., et al., Functional significance of olfactory cells of a snakehead Ophiocephalus striatus (Bloch), Bionano Front., 2012, vol. 5, no. 2, pp. 145–148.
Kim, B.H.T., Kim, H.S., and Park, J.Y., The Anatomy and histology of the olfactory organ in the Korean sand goby Favonigobius gymnauchen (Pisces, Gobiidae), Kor. J. Ichthyol., 2016, vol. 28, no. 1, pp. 28–34.
Kramer, D. and Chapman, M., Implications of fish home range size and relocation for marine reserve function, Environ. Biol. Fish., 1999, vol. 55, pp. 65–79. https://doi.org/10.1023/A:1007481206399
Kramer, M.J., Bellwood, O., Fulton, C.J., and Bellwood, D.R., Refining the invertivore: diversity and specialization in fish predation on coral reef crustaceans, Mar. Biol., 2015, vol. 162, pp. 1779–1786. https://doi.org/10.1007/s00227-015-2710-0
Kuciel, M., Zuwała, K., and Jakubowski, M., A new type of fish olfactory organ structure in Periophthalmus barbarus (Oxudercinae), Acta Zool., 2011, vol. 92, pp. 276–280. https://doi.org/10.1111/j.1463-6395.2010.00459.x
Kuciel, M., Zuwała, K., and Satapoominb, U., Comparative morphology (SEM) of the peripheral olfactory organ in the Oxudercinae subfamily (Gobiidae, Perciformes), Zool. Anz., 2013, vol. 252, no. 4, pp. 424–430. https://doi.org/10.1016/j.jcz.2013.03.002
Lara, M.R., Morphology of the eye and visual acuities in the settlement-intervals of some coral reef fishes (Labridae, Scaridae), Environ. Biol. Fish., 2001, vol. 62, pp. 365–378. https://doi.org/10.1023/A:1012214229164
Lara, M.R., Development of the nasal olfactory organs in the larvae, settlement-stages and some adults of 14 species of Caribbean reef fishes (Labridae, Scaridae, Pomacentridae), Mar. Biol., 2008, vol. 154, no. 1, pp. 51–64. https://doi.org/10.1007/s00227-007-0899-2
Lecchini, D. and Nakamura, Y., Use of chemical cues by coral reef animal larvae for habitat selection, Aquat. Biol., 2013, vol. 19, pp. 231–238. https://doi.org/10.3354/ab00532
Lecchini, D., Osenberg, C.W., Shima, J.S., et al., Ontogenetic changes in habitat selection during settlement in a coral reef fish: ecological determinants and sensory mechanisms, Coral Reefs, 2007, vol. 26, pp. 423–432. https://doi.org/10.1007/s00338-007-0212-3
Lek, E., Fairclough, D.V., Platell, M.E., et al., To what extents are the dietary compositions of three abundant, co-occurring labrid species different and related to latitude, habitat, body size and season? J. Fish Biol., 2011, vol. 78, pp. 1913–1943. https://doi.org/10.1111/j.1095-8649.2011.02961.x
Lek, E., Platell, M.E., Fairclough, D.V., et al., Diets of reef-dwelling labrids (Choerodon species) vary with body size, season and habitat: influence of foraging ability, specialization and opportunism, J. Fish Biol., 2018, vol. 92, pp. 901–928. https://doi.org/10.1111/jfb.13541
Lieske, E. and Myers, R., Coral Reef Fishes: Indo-Pacific & Caribbean (Collins Pocket Guide), London: HarperCollins, 1994.
Losey, G.S., Cronin, T., Goldsmith, T.H., et al., The UV visual world of fishes: a review, J. Fish Biol., 1999, vol. 54, pp. 921–943. https://doi.org/10.1006/jfbi.1998.0919
Mana, R.R. and Kawamura, G., Olfactory organs of two pelagic teleost fish–opah (Lampris guttatus) and dolphin fish (Coryphaena hippurus), S. Pac. Study, 2002, vol. 22, no. 2, pp. 53–64.
Mandal, D.K., Roy, D., and Ghosh, L., Structural organization of the olfactory epithelium of a spotted snakehead fish, Channa punctatus, Acta Ichthyol. Piscat., 2005, vol. 35, no. 1, pp. 45–50.
Marshall, N.J., Communication and camouflage with the same ‘bright’ colors in reef fishes, Philos. Trans. R. Soc. B, 2000, vol. 355, pp. 1243–1248. https://doi.org/10.1098/rstb.2000.0676
Marty, M.J., Blum, J.E., and Pawlik, J.R., No accounting for taste: palatability of variably defended Caribbean sponge species is unrelated to predator abundance, J. Exp. Mar. Biol. Ecol., 2016, vol. 485, pp. 57–64. https://doi.org/10.1016/j.jembe.2016.08.014
Masterson, C.F., Danilowicz, B.S., and Sale, P.F., Yearly and inter-island variation in the recruitment dynamics of the bluehead wrasse (Thalassoma bifasciatum, Bloch), J. Exp. Mar. Biol. Ecol., 1997, vol. 214, pp. 149–166. https://doi.org/10.1016/S0022-0981(97)00020-8
McClintock, J.B., Baker, B.J., Baumiller, T.K., and Messing, C.G., Lack of chemical defense in two species of stalked crinoids: support for the predation hypothesis for Mesozoic bathymetric restriction, J. Exp. Mar. Biol. Ecol., 1999, vol. 232, pp. 1–7. https://doi.org/10.1016/S0022-0981(98)00003-3
Michiels, N.K., Anthes, N., Hart, N.S., et al., Red fluorescence in reef fish: a novel signaling mechanism? BMC Ecol., 2008, vol. 8, p. 16. https://doi.org/10.1186/1472-6785-8-16
Miller, A.M. and Pawlik, J.R., Do coral reef fish learn to avoid unpalatable prey using visual cues? Anim. Behav., 2013, vol. 85, no. 2, pp. 339–347. https://doi.org/10.1016/j.anbehav.2012.11.002
Mochek, A.D., Etologicheskaya organizatsiya pribrezhnykh soobshchestv morksikh ryb (Ethological Organization of Coastal Marine Fish Communities), Moscow: Nauka, 1987.
Moland, E., Eagle, J.V., and Jones, G.P., Ecology and evolution of mimicry in coral reef fishes, Oceanogr. Mar. Biol., 2005, vol. 43, pp. 455–482.
Morton, J., Platell, M., and Gladstone, W., Differences in feeding ecology among three co-occurring species of wrasse (Teleostei: Labridae) on rocky reefs of temperate Australia, Mar. Biol., 2008, vol. 154, pp. 577–592. https://doi.org/10.1007/s00227-008-0951-x
Nagel, L. and Grutter, A.S., Host preference and specialization in Gnathia sp., a common parasitic isopod of coral reef fishes, J. Fish Biol., 2007, vol. 70, pp. 497–508. https://doi.org/10.1111/j.1095-8649.2007.01320.x
Nelson, J.S., Grande, T.C., and Wilson, M.V.H., Fishes of the World, Hoboken, NJ: Wiley, 2016.
Parin, N.V., Family Labridae, in Zhizn’ zhivotnykh. Tom 4. Chast’ 1. Ryby (The Life of Animals, Vol. 4, Part 1: Fishes), Rass, T.S., Ed., Moscow: Prosveshchenie, 1971, pp. 491–492.
Pashchenko, N.I. and Kasumyan, A.O., Some morphofunctional features of development of olfactory organ in ontogenesis of a minnow, Zool. Zh., 1983, vol. 62, no. 3, pp. 367–377.
Pashchenko, N.I. and Kasumyan, A.O., Morphofunctional features of development of olfactory organ of carps (Cypriniformes, Cyprinidae). I. Morphology and functions of olfactory organs in ontogenesis of the grass carp Ctenopharyngodon idella (Val.), Vopr. Ikhtiol., 1986, vol. 26, no. 2, pp. 303–317.
Pashchenko, N.I. and Kasumyan, A.O., Scanning electron microscopy of development of the olfactory organ in ontogeny of grass carp Ctenopharyngodon idella, J. Ichthyol., 2015, vol. 55, no. 6, pp. 880–899. https://doi.org/10.1134/S0032945215060132
Pashchenko, N.I. and Kasumyan, A.O., Development of the olfactory organ in the ontogeny of carps (Cyprinidae), J. Ichthyol., 2017, vol. 57, no. 1, pp. 136–151. https://doi.org/10.1134/S0032945217010088
Paśko, Ł., Tool-like behavior in the sixbar wrasse, Thalassoma hardwicke (Bennett, 1830), Zoo Biol., 2010, vol. 29, pp. 767–773.
Pawlik, J.R., Chanas, B., Toonen, R.J., and Fenical, W., Defenses of Caribbean sponges against predatory reef fish. 1. Chemical deterrency, Mar. Ecol.: Prog. Ser., 1995, vol. 127, pp. 183–194. https://doi.org/10.3354/meps127183
Price, S.A., Holzman R., Near T.J., and Wainwright, P.C., Coral reefs promote the evolution of morphological diversity and ecological novelty in labrid fishes, Ecol. Lett., 2011, vol. 14, pp. 462–469. https://doi.org/10.1111/j.1461-0248.2011.01607.x
Randall, J.E., A review of mimicry in marine fishes, Zool. Stud., 2005, vol. 44, no. 3, pp. 299–328.
Randall, J.E., Allen, G.R., and Steene, R.C., Fishes of the Great Barrier Reef and Coral Sea, Bathurst: Crawford, 1997.
Robertson, D.R., Who resembles whom? Mimetic and coincidental look-alikes among tropical reef fishes, PLoS One, 2013, vol. 8, no. 1, art. ID e54939. https://doi.org/10.1371/journal.pone.0054939
Rocha, R.A. and Bowen, B.W., Speciation in coral-reef fishes, J. Fish Biol., 2008, vol. 72, pp. 1101–1121. https://doi.org/10.1111/j.1095-8649.2007.01770.x
Sarkar, S.K., Acharya, A., Jana, S., and De, S.K., Macro-anatomical variation of the olfactory apparatus in some Indian teleosts with special reference to their ecological habitat, Folia Morphol., 2014, vol. 73, no. 2, pp. 122–128. https://doi.org/10.13140/RG.2.1.4362.5124
Schubert, M., Munday, P.L., Caley, M.J., et al., The toxicity of skin secretions from coral-dwelling gobies and their potential role as a predator deterrent, Environ. Biol. Fish., 2003, vol. 67, pp. 359–367. https://doi.org/10.1023/A:1025826829548
Schuijf, A., Baretta, J.W., and Wildschut, J.T., A field investigation on the discrimination of sound direction in Labrus bergylta (Pisces: Perciformes), Neth. J. Zool., 1972, vol. 22, pp. 81–104.
Shepherd, S.A. and Clarkson, P.S., Diet, feeding behavior, activity and predation of the temperate blue-throated wrasse, Notolabrus tetricus, Mar. Freshwater Res., 2001, vol. 52, pp. 311–322. https://doi.org/10.1071/MF99040
Siebeck, U.E. and Marshall, N.J., Transmission of ocular media in labrid fishes, Philos. Trans. R. Soc. B, 2000, vol. 355, pp. 1257–1261. https://doi.org/10.1098/rstb.2000.0679
Siebeck, U.E. and Marshall, N.J., Ocular media transmission of coral reef fish–can coral reef fish see ultraviolet light? Vision Res., 2001, vol. 41, pp. 133–149. https://doi.org/10.1016/S0042-6989(00)00240-6
Sinha, S.K. and Sinha, R.K., Morphology and the anatomy of the olfactory organs of the marine fish Thynnus thunnina (Cuv. et Val.), Folia Morphol., 1990, vol. 38, no. 2, pp. 169–173.
Slamet, B. and Hutapea, J.H., First successful hatchery production of Napoleon wrasse at Gondol Research Institute for Mariculture, Bali, S. Pac. Comm. Live Reef Fish Inf. Bull., 2005, vol. 13, pp. 43–44.
Sollid, J., De Angelis, P., Gundersen, K., and Nilsson, G.E., Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills, J. Exp. Biol., 2003, vol. 206, pp. 3667–3673. https://doi.org/10.1242/jeb.00594
Sollid, J., Weber, R.E., and Nilsson, G.E., Temperature alters the respiratory surface area of crucian carp Carassius carassius and goldfish Carassius auratus, J. Exp. Biol., 2005, vol. 208, pp. 1109–1116. https://doi.org/10.1242/jeb.01505
Sponaugle, S. and Cowen, R.K., Early life history traits and recruitment patterns of Caribbean wrasses (Labridae), Ecol. Monogr., 1997, vol. 67, pp. 177–202.
Status of Coral Reefs of the World: 2008, Wilkinson, C., Ed., Townsville: Global Coral Reef Monit. Network, 2008.
Steinberg, J.C., Cummings, W.C., Brahy, B.D., and MacBain, J.Y., Further bio-acoustic studies off the west coast of north Bimini, Bahamas, Bull. Mar. Sci., 1965, vol. 15, pp. 942–963.
Stier, A.C. and White, J.W., Predator density and the functional responses of coral reef fish, Coral Reefs, 2014, vol. 33, pp. 235–240. https://doi.org/10.1007/s00338-013-1096-z
Streelman, J.T. and Karl, S.A., Reconstructing labroid evolution with single-copy nuclear DNA, Proc. R. Soc. B, 1997, vol. 264, pp. 1011–1020. https://doi.org/10.1098/rspb.1997.0140
Suzuki, S., Kuwamura, T., Nakashima, Y., et al., Social factors of group spawning as an alternative mating tactic in the territorial males of the threespot wrasse Halichoeres trimaculatus, Environ. Biol. Fish., 2010, vol. 89, pp. 71–77. https://doi.org/10.1007/s10641-010-9691-0
Sweatman, H.P.A., Field evidence that settling coral reef fish larvae detect resident fishes using dissolved chemical cues, J. Exp. Mar. Biol. Ecol., 1988, vol. 124, pp. 163–174. https://doi.org/10.1016/0022-0981(88)90170-0
Tavolga, W.N. and Wodinsky, J., Auditory capacities in fishes: pure tone thresholds in nine species of marine teleosts, Bull. Am. Mus. Nat. Hist., 1963, vol. 126, pp. 97–115.
Tkachenko, K.S., Coral reefs in the face of ecological threats of the 21st century, Biol. Bull. Rev., 2017, vol. 7, no. 1, pp. 64–83.
Topping, D.T., Lowe, C.G., and Caselle, J.E., Home range and habitat utilization of adult California sheephead, Semicossyphus pulcher (Labridae), in a temperate no-take marine reserve, Mar. Biol., 2005, vol. 147, pp. 301–311. https://doi.org/10.1007/s00227-005-1573-1
Tricas, T.C. and Boyle, K.S., Acoustic behaviors in Hawaiian coral reef fish communities, Mar. Ecol.: Prog. Ser., 2014, vol. 511, pp. 1–16. https://doi.org/10.3354/meps10930
Vail, A.L. and McCormick, M.I., Metamorphosing reef fishes avoid predator scent when choosing a home, Biol. Lett., 2011, vol. 7, pp. 921–924. https://doi.org/10.1098/rsbl.2011.0380
Victor, B.C., Duration of the planktonic larval stage of one hundred species of Pacific and Atlantic wrasses (family Labridae), Mar. Biol., 1986, vol. 90, pp. 317–326. https://doi.org/10.1007/BF00428555
Wainwright, P.C., Morphology and ecology: functional basis of feeding constraints in Caribbean labrid fishes, Ecology, 1988, vol. 69, pp. 635–645.
Wainwright, P.C., Bellwood, D.R., and Westneat, M.W., Ecomorphology of locomotion in labrid fishes, Environ. Biol. Fish., 2002, vol. 65, pp. 47–62. https://doi.org/10.1023/A:1019671131001
Wainwright, P.C., Bellwood, D.R., Westneat, M.W., et al., A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system, Biol. J. Linn. Soc., 2004, vol. 82, pp. 1–25. https://doi.org/10.1111/j.1095-8312.2004.00313.x
Walker, J.A. and Westneat, M.W., Performance limits of labriform propulsion and correlates with fin shape and motion, J. Exp. Biol., 2002, vol. 205, pp. 177–187.
Warner, R.R., The role of extreme iteroparity and risk avoidance in the evolution of mating systems, J. Fish Biol., 2005, vol. 53, suppl. A, pp. 82–93. https://doi.org/10.1111/j.1095-8649.1998.tb01019.x
Westneat, M.W., Phylogenetic relationships of the tribe Cheilinini (Labridae: Perciformes), Bull. Mar. Sci., 1993, vol. 52, pp. 351–394.
Westneat, M.W. and Alfaro, M.E., Phylogenetic relationships and evolutionary history of the reef fish family Labridae, Mol. Phylogenet. Evol., 2005, vol. 36, pp. 370–390. https://doi.org/10.1016/j.ympev.2005.02.001
Westneat, M.W., Alfaro, M.E., Wainwright, P.C., et al., Local phylogenetic divergence and global evolutionary convergence of skull function in reef fishes of the family Labridae, Proc. R. Soc. B, 2005, vol. 272, pp. 993–1000. https://doi.org/10.1098/rspb.2004.3013
White, J.W. and Warner, R.R., Behavioral and energetic costs of group membership in a coral reef fish, Oecologia, 2007, vol. 154, pp. 423–433. https://doi.org/10.1007/s00442-007-0838-4
Winn, H.E., Formation of a mucous envelope at night by parrot fishes, Zoologica, 1955, vol. 40, pp. 145–148.
Winn, H.E. and Bardach, J.E., Differential food selection by moray eels and a possible role of the mucous envelope of parrot fishes in reduction of predation, Ecology, 1959, vol. 40, pp. 296–298.
Yaakub, S.M., Bellwood, D.R., van Herwerden, L., and Walsh, F.M., Hybridization in coral reef fishes: introgression and bi-directional gene exchange in Thalassoma (family Labridae), Mol. Phylogenet. Evol., 2006, vol. 40, pp. 84–100. https://doi.org/10.1016/j.ympev.2006.02.012
Yamamoto, M., Comparative morphology of fish olfactory organ in teleosts, in Chemoreception in Fishes, Hara, T.J., Ed., New York: Elsevier, 1982, pp. 39–59.
Yamamoto, M. and Ueda, K., Comparative morphology of fish olfactory epithelium. X. Perciformes, Beryciformes, Scorpaeniformes, and Pleuronectiformes, J. Fac. Sci. Tokyo Univ., 1979, vol. 14, pp. 273–297.
Zeiske, E., Theisen, B., and Breucker, H., Structure, development, and evolutionary aspects of the peripheral olfactory system, in Fish Chemoreception, Fish Fish. Ser., vol 6, Hara, T.J., Ed., Dordrecht: Springer-Verlag, 1992, pp. 13–39. https://doi.org/10.1007/978-94-011-2332-7_2
Funding
The collection of study materials was supported by the Joint Russian-Vietnamese Tropical Research and Technology Center. The experimental part of the study, primary data analysis, analysis of the results, and article writing were conducted as part of the Moscow State University project Noah’s Ark and Russian Foundation for Basic Research (project no. 19–04–00367).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by E. Sherstyuk
Rights and permissions
About this article
Cite this article
Pashchenko, N.I., Kasumyan, A.O. & Oanh, L.T. Atypical Structure of Olfactory Organ in Moon Wrasse Thalassoma lunare and Sixbar Wrasse T. hardwicke (Labridae). J. Ichthyol. 61, 348–360 (2021). https://doi.org/10.1134/S0032945221030073
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945221030073