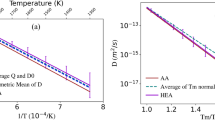
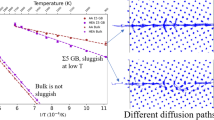
Abstract
The molecular dynamics method is used to provide fundamental insights into surface segregation, bulk diffusion and alloying reaction phenomena in equiatomic Ni-Al systems. This knowledge can serve as a guide for the search and development of economic routes for controlling microstructure and properties of the intermetallic compound NiAl. This paper gives an overview of recent molecular dynamics simulations in the area along with other theoretical calculations and experimental measurements.
Similar content being viewed by others
References
D. B. Miracle, “The Physical and Mechanical Properties of NiAl,” Acta Metall. Mater. 41, 649–684 (1993).
Intermetallic Compounds: Structural Applications, Vol. 4, ed. by J. H. Westbrook and R. L. Fleischer (Wiley, New York, 2000).
H.-J. Freund, H. Kuhlenbeck, and V. Staemmler, “Oxide Surfaces,” Rep. Prog. Phys. 59, 283–347, (1996).
M. W. Finnis, A. Y. Lozovoi, and A. Alavi, “The Oxidation of NiAl: What Can We Learn from Ab Initio Calculations?” Ann. Rev. Mater. Res. 35, 167–207, (2005).
J. A. Brown and Y. Mishin, “Monte Carlo Modeling of Low-Index Surfaces in Stoichiometric and Ni-Rich NiAl,” Phys. Rev. B: Condens. Matter Mater. Phys. 67, 195414 (2003).
J. A. Brown and Y. Mishin, “Effect of Surface Stress on Ni Segregation in (110) NiAl Thin Films,” Phys. Rev. B: Condens. Matter Mater. Phys. 69, 195407 (2004).
A. J. Bradley and A. Taylor, “An X-ray Analysis of the Nickel-Aluminum System,” Proc. R. Soc. Lond. Ser. A 159, 56–72 (1937).
A. Taylor and N. J. Doyle, “Further Studies on the Nickel-Aluminum System. I. β-NiAl and δ-Ni2Al3 Phase Fields,” J. Appl. Crystallogr. 5, 201–209 (1972).
B. Meyer and M. Fähnle, “Atomic Defects in the Ordered Compound B2-NiAl: A Combination of Ab Initio Electron Theory and Statistical Mechanics,” Phys. Rev. B: Condens. Matter Mater. Phys. 59, 6072–6082 (1999).
P. A. Korzhavyi, A. V. Ruban, A. Y. Lozovoi, Y. K. Vekilov, I. A. Abrikosov, and B. Johanson, “Constitutional and Thermal Point Defects in B2 NiAl,” Phys. Rev. B: Condens. Matter Mater. Phys. 61, 6003–6018 (2000).
A. Y. Lozovoi and Y. Mishin, “Point Defects in NiAl: The Effect of Lattice Vibrations,” Phys. Rev. B: Condens. Matter Mater. Phys. 68, 184113 (2003).
H. L. Davis and J. R. Noonan, “Rippled Relaxation in the (110) Surface of the Ordered Metallic Alloy NiAl,” Phys. Rev. Lett. 54, 566–569 (1985).
S. M. Yalisove and W. R. Graham, “Multilayer Rippled Structure of the NiAl(110) Surface: A Medium Energy Ion Scattering Study,” Surf. Sci. 183, 556–564 (1987).
K. F. McCarty, J. A. Nobel, and N. C. Bartlet, “Vacancies in Solids and the Stability of Surface Morphology,” Nature 412, 622–625 (2001).
K. F. McCarty, J. A. Nobel, and N. C. Bartlet, “Surface Dynamics Dominated by Bulk Thermal Defects: The Case of NiAl(110).” Phys. Rev. B: Condens. Matter Mater. Phys. 71, 085421 (2005).
J. R. Noonan and H. L. Davis, “Mixture of Ordered Domains in the NiAl(111) Surface,” Phys. Rev. Lett. 59, 1714–1717 (1987).
D. R. Mullins and S. H. Overbury, “The Structure and Composition of the NiAl(110) and NiAl(100) Surfaces,” Surf. Sci. 199(1–2), 141–153 (1988).
S.A. Chambers, “Surface Termination of Epitaxial NiAl on GaAs(001) by High-Angular-Resolution X-ray Photoelectron Diffraction,” Phys. Rev. B: Condens. Matter Mater. Phys. 42, 10865–10872 (1990).
W. D. Roos, J. du Plessis, G. N. van Wyk, E. Taglauer, and S. Wolf, “Surface Structure and Composition of NiAl(100) by Low-Energy Ion Scattering,” J. Vac. Sci. Technol. A 14, 1648–1651 (1996).
M. H. Yoo and C. L. Fu, “On the Theory of Cleavage Fracture in B2-Type Aluminides—FeAl and NiAl,” Scripta Metall. Mater. 25, 2345–2350 (1991).
A. T. Hanbicki, A. P. Baddorf, E. W. Plummer, B. Hammer, and M. Scheffler, “The Interaction of Hydrogen with the (110) Surface of NiAl,” Surf. Sci. 331–333,Part A, 811–817 (1995).
N. I. Medvedeva, O. N. Mryasov, Y. N. Gornostyrev, D. L. Novikov, and A. J. Freeman, “First-Principles Total-Energy Calculations for Planar Shear and Cleavage Decohesion Processes in B2-Ordered NiAl and FeAl,” Phys. Rev. B: Condens. Matter Mater. Phys. 54, 13506–13514 (1996).
A. Y. Lozovoi, A. Alavi, and M. W. Finnis, “Surface Stoichiometry and the Initial Oxidation of NiAl(110),” Phys. Rev. Lett. 85, 610–613 (2000).
Y. Mishin, M. J. Mehl, and D. A. Papaconstantopoulos, “Embedded-Atom Potential for B2-NiAl,” Phys. Rev. B: Condens. Matter Mater. Phys. 65, 224114 (2002).
R. Bohn, T. Haubold, R. Birringer, and H. Gleiter, Nanocrystalline Intermetallic Compounds an Approach to Ductility?” Scripta Metall. 25, 811–816 (1991).
M. Fukumoto, M. Yamasaki, M. Nie, and T. Yasui, “Synthesis and Characterization of Nano-Structured NiAl Intermetallic Compound Coating.” Quart. J. Jpn. Weld. Soc. 24, 87–92 (2006).
T Shibata, B.A. Bunker, Z.Y. Zhang, D. Meisel, C. F. Vardeman, and J. D. Gezelter, “Size Dependent Spontaneous Alloying of Au-Ag Nanoparticles,” J. Am. Chem. Soc. 124, 11989–11996 (2002).
S. Dong, P. Hou, H. Cheng, H. Yang, and G. Zou, “Fabrication of Intermetallic NiAl by Self-Propagating High-Temperature Synthesis Reaction Using Aluminum Nanopowder under High Pressure,” J. Phys.: Condens. Matter 14, 11023–11030 (2002).
Y. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai, and A. P. Alivisatos, “Formation of Hollow Nanocrystals through the Nanoscale Kirkendall Effect,” Science 304, 711–714 (2004).
A. Kovács and Y. Hirotsu, “Fabrication of L10-PdCoFe Nanocrystalline Particles with Tilted Magnetic Easy Axis,” Appl. Phys. Lett. 91, 193106 (2007).
Y.L. Chueh, A.C. Ford, J.C. Ho, Z.A. Jacobson, Z. Fan, C.Y. Chen, L.J. Chou, and A. Javey, “Formation and Characterization of NixInAs/InAs Nanowire Heterostructures by Solid Source Reaction,” Nano Lett. 8, 4528–4533 (2008).
R. Nakamura, G. Matsubayashi, H. Tsuchiya, S. Fujimoto, and H. Nakajima, “Formation of Oxide Nanotubes via Oxidation of Fe, Cu and Ni Nanowires and Their Structural Stability: Difference in Formation and Shrinkage Behavior of Interior Pores,” Acta Mater. 57, 5046–5052 (2009).
E. M. Hunt, K. B. Pantier, and M. L. Pantoya, “Nano-Scale Reactants in the Self-Propagating High-Temperature Synthesis of Nickel Aluminide,” Acta Mater. 52, 3183–3191 (2004).
S. O. Moussa and M. S. El-Shall, “Fabrication of Nanostructured Nickel and Titanium Aluminides Starting from Elemental Nanopowders,” Mater. Chem. Phys. 112, 1015–1020 (2008).
J. Philibert, “Reactive Diffusion in Thin Films,” Appl. Surf. Sci. 53, 74–81 (1991).
H. Mehrer, “Diffusion in Intermetallics,” Mater. Trans. Jpn. Inst. Metals 37, 1259–1280 (1996).
H. J. Fan, U. Gösele, and M. Zacharias, “Nanotubes and Hollow Nanoparticles Based on Kirkendall and Diffusion Process: A Review,” Small 3, 1660–1671 (2007).
G. Shmitz, C.-B. Ene, and C. Nowak, Reactive Diffusion in Nanostructures of Spherical Symmetry,” Acta Mater. 57, 2673–2683 (2009).
S. Yip, Handbook of Materials Modeling (Springer-Verlag, Dordrecht, 2005).
S. Zhao, T. C. Germann, and A. Strachan, “Atomistic Simulations of Shock-Induced Alloying Reactions in Ni/Al Nanolaminates,” J. Chem. Phys. 125, 164707 (2006).
S. Zhao, T. C. Germann, and A. Strachan, “Melting and Alloying of NiAl Nanolaminates Induced by Shock Loading: A Molecular Dynamics Simulation Study,” Phys. Rev. B: Condens. Matter Mater. Phys. 76, 104105 (2007).
B. J. Henz, T. Hawa, and M. Zachariah, Molecular Dynamics Simulation of the Kinetic Sintering of Ni and Al Nanoparticles,” Mol. Simulat. 35, 804–811 (2009).
B. J. Henz, T. Hawa, and M. Zachariah, “Molecular Dynamics Simulation of the Kinetic Reaction between Ni and Al Nanoparticles,” J. Appl. Phys. 105, 124310 (2009).
A. V. Evteev, E. V. Levchenko, I. V. Belova, and G. E. Murch, “Interdiffusion and Surface-Sandwich Ordering in Initial Ni-Core-Pd-Shell Nanoparticle,” Phys. Chem. Chem. Phys. 11, 3233–3340 (2009).
A. V. Evteev, E. V. Levchenko, D. P. Riley, I. V. Belova, and G. E. Murch, “Reaction of a Ni-Coated Al Nanoparticle to Form B2-NiAl: A Molecular Dynamics Study.” Phil. Mag. Lett. 89, 815–830 (2009).
W. H. Qi and S. T. Lee, “Phase Stability, Melting and Alloy Formation of Au-Ag Bimetallic Nanoparticles,” J. Phys. Chem. C 114, 9580–9587 (2010).
E. V. Levchenko, A. V. Evteev, D. P. Riley, I. V. Belova, and G. E. Murch, “Molecular Dynamics Simulation of the Alloying Reaction in Al-Coated Ni Nanoparticles,” Comput. Mater. Sci. 47, 712–720 (2009).
N. H. Nguyen, A. Hu, J. Persic, and J. Z. Wen, “Molecular Dynamics Simulation of Energetic Aluminum/Palladium Core-Shell Nanoparticles,” Chem. Phys. Lett. 503, 112–117 (2011).
A. V. Evteev, E. V. Levchenko, F. A. Hagel, I. V. Belova, and G. E. Murch, “Molecular Dynamics Study of Reaction Pathways in an Al-Coated Ni Nanoparticle,” Intermetallics 19, 934–941 (2011).
F. Delogu, “Ignition of an Exothermal Reaction by Collision between Al and Ni Crystals,” J. Appl. Phys. 110, 103505 (2011).
E. V. Levchenko, A. V. Evteev, G. G. Löwisch, I. V. Belova, and G. E. Murch, “Molecular Dynamics Simulation of Alloying in a Ti-Coated Al Nanoparticle,” Intermetallics 22, 193–202 (2012).
J. Hafner, “Atomic-Scale Computational Materials Science,” Acta Mater. 48, 71–92 (2000).
M. S. Daw and M. I. Baskes, “Embedded-Atom Method: Derivation and Application to Impurities, Surfaces, and Other Defects in Metals,” Phys. Rev. B: Condens. Matter Mater. Phys. 29, 6443–6453 (1984).
E. V. Levchenko, A. V. Evteev, I. V. Belova and G. E. Murch, “Molecular Dynamics Study of Density, Surface Energy and Self-Diffusion in a Liquid Ni50Al50 Alloy,” Comput. Mater. Sci. 50, 331–337 (2010).
E. V. Levchenko, A. V. Evteev, D. R. Beck, I. V. Belova, and G. E. Murch, “Molecular Dynamics Simulation of the Thermophysical Properties of an Under-Cooled Liquid NiAl Alloy,” Comput. Mater. Sci. 50, 465–473 (2010).
E. V. Levchenko, A. V. Evteev, R. Kozubski, I. V. Belova, and G. E. Murch, “Molecular Dynamics Simulation of Surface Segregation in a (110) B2-NiAl Thin Film, Phys. Chem. Chem. Phys. 13, 1214–1221 (2011).
A. V. Evteev, E. V. Levchenko, I. V. Belova, and G. E. Murch, “Molecular Dynamics Simulation of Diffusion in a (110) B2-NiAl Film,” Intermetallics 19, 848–854 (2011).
A. V. Evteev, E. V. Levchenko, I. V. Belova and G. E. Murch, “Molecular Dynamics Assessment of the Time-Temperature-Transformation Diagram for Crystallization of an Undercooled Liquid Ni50Al50 Alloy, Acta Mater. 59, 6412–6419 (2011).
L. Verlet, “Computer “Experiments” on Classical Fluids. I. Thermodynamical Properties of Lennard-Jones Molecules,” Phys. Rev. 159, 98–203 (1967).
Y. Mishin, Diffusion Processes in Advanced Technological Materials Ed. by D. Gupta (William Andrew Norwich, New York, 2005), p. 113.
X. Xie and Y. Mishin, “Monte Carlo Simulation of Grain Boundary Segregation and Decohesion in NiAl.” Acta Mater. 50, 4303–4313 (2002).
A. Kerrache, J. Horbach, and K. Binder, “Molecular-Dynamics Computer Simulation of Crystal Growth and Melting in Al50Ni50,” Europhys. Lett. 81, 58001 (2008).
Y. Mishin, A. Y. Lozovoi, and A. Alavi, “Evaluation of Diffusion Mechanisms in NiAl by Embedded-Atom and First-Principles Calculations,” Phys. Rev. B: Condens. Matter Mater. Phys. 67, 014201 (2003).
M. I. Mendelev and Y. Mishin, “Molecular Dynamics Study of Self-Diffusion in BCC Fe,” Phys. Rev. B: Condens. Matter Mater. Phys. 80, 144111 (2009).
G. J. Ackland and M. W. Finnis, “Semi-Empirical Calculation of Solid Surface Tensions in BCC Transition Metals,” Phil. Mag. A 54, 301–315 (1986).
P. Gumbsch and M. S. Daw, “Interface Stresses and Their Effects on the Elastic Moduli of Metallic Multilayers,” Phys. Rev. B: Condens. Matter 44, 3934–3938 (1991).
L. E. Murr, Interfacial Phenomena in Metals and Alloys (Addison-Wesley, Reading, MA, 1975).
St. Frank, S. V. Divinski, U. Södervall, and Chr. Herzig, “Ni Tracer Diffusion in the B2-Compound NiAl: Influence of Temperature and Composition,” Acta Mater. 49, 1399–1411 (2001).
N. A. Stolwijk, M. van Gend and H. Bakker, “Self-Diffusion in the Intermetallic Compound CoGa,” Phil. Mag. A 42,783–808 (1980).
E. W. Elcock and C. W. McCombie, “Vacancy Diffusion in Binary Ordered Alloys,” Phys. Rev. 109, 605–606 (1958).
M. Arita, M. Koiwa and S. Ishioka, “Diffusion Mechanisms in Ordered Alloys-a Detailed Analysis of Six-Jump Vacancy Cycle in the B2 Type Lattice.” Acta Metall. 37, 1363–1374 (1989).
H. Hahn, G. Frohberg and H. Wever, “Self Diffusion in the Intermetallic B2 Electron Compound PdIn,” Phys. Status Solidi A 79, 559–565 (1983).
G. F. Hancock and B. R. McDonnel, “Diffusion in the Intermetallic Compound NiAl,” Phys. Status Solidi A 4, 143–150 (1971).
K. A. Marino and E. A. Carter, “First-Principles Characterization of Ni Diffusion Kinetics in β-NiAl,” Phys. Rev. B: Condens. Matter Mater. Phys. 78, 184105 (2008).
K. A. Marino and E. A. Carter, “The Effect of Platinum on Al Diffusion Kinetics in β-NiAl: Implications for Thermal Barrier Coating Lifetime,” Acta Mater. 58, 2726–2737 (2010).
G. H. Vineyard, “Frequency Factors and Isotope Effects in Solid State Rate Processes,” J. Phys. Chem. Solids 3, 121–127 (1957).
Q. Xu and A. van der Ven, “Atomic Transport in Ordered Compounds Mediated by Local Disorder: Diffusion in B2-NixAl1 − x ,” Phys. Rev. B: Condens. Matter Mater. Phys. 81, 064303 (2010).
M. I. Mendelev and Y. Mishin, “Molecular Dynamics Study of Self-Diffusion in BCC Fe,” Phys. Rev. B: Condens. Matter Mater. Phys. 80, 144111 (2009).
M. I. Mendelev and B. S. Bokstein, “Molecular Dynamics Study of Self-Diffusion in Zr,” Phil. Mag. B 90, 637–654 (2010).
H. Mehrer, Diffusion in Solids (Springer-Verlag, Berlin, 2007).
R. Drautz and M. Fähnle, “The Six-Jump Diffusion Cycles in B2 Compounds Revisited,” Acta Mater. 47, 2437–2447 (1999).
I. V. Belova and G. E. Murch, “A Theory of Tracer Diffusion in Nonstoichiometric Intermetallic Compounds, Phil. Mag. A 82, 269–283 (2002).
Proceedings of the Int. Symp. on Nickel and Iron Aluminides: Processing, Properties, and Applications, Ed. by S. C. Deevi, P. J. Maziasz, V. K. Sikka, and R. W. Cahn, (ASM International, Metals Park, OH, 1997).
S. C. Deevi, D. G. Morris, and V. K. Sikka, “Preface,” Mater. Sci. Eng. A 258, xi (1998).
S. Stüber, D. Holland-Moritz, T. Unruh, and A. Meyer, “Ni Self-Diffusion in Refractory Al-Ni Melts,” Phys. Rev. B: Condens. Matter Mater. Phys. 81, 024204 (2010).
D. M. Herlach, R. Lengsdorf, P. Galenko, H. Hartmann, C.-A. Gandin, S. Mosbah, A. Garcia-Escorial, and H. Henein, “Non-Equilibrium and Near-Equilibrium Solidification of Undercooled Melts of Ni- and Al-Based Alloys,” Adv. Eng. Mater. 10, 444–452 (2008).
I. Egry and J. Brillo, “Surface Tension and Density of Liquid Metallic Alloys Measured by Electromagnetic Levitation,” J. Chem. Eng. Data 54, 2347–2352 (2009).
J. W. Cahn, in Interface Segregation, Ed. by W. C. Johnson and J. M. Blackely (American Society for Metals, Metals Park, OH, 1979), Ch. 1, p. 3.
T. Frolov and Y. Mishin, “Temperature Dependence of the Surface Free Energy and Surface Stress: An Atomistic Calculation for Cu(110).” Phys. Rev. B: Condens. Matter Mater. Phys. 79, 045430 (2009).
S. Reutzel, H. Hartmann, P. K. Galenko, S. Schneider, and D. M. Herlach, “Change of the Kinetics of Solidification and Microstructure Formation Induced by Convection in the Ni-Al System,” Appl. Phys. Lett. 91, 041913 (2007).
Y. Plevachuk, I. Egry, J. Brillo, D. Holland-Moritz, and I. Kaban, “Density and Atomic Volume in Liquid Al-Fe and Al-Ni Binary Alloys,” Int. J. Mater. Res. 98, 107–111 (2007).
I. Egry, J. Brillo, D. Holland-Moritz, and Y. Plevachuk, “The Surface Tension of Liquid Aluminum-Based Alloys,” Mater. Sci. Eng. A 495, 14–18 (2008).
S. K. Das, J. Horbach, and T. Voigtmann, “Structural Relaxation in a Binary Metallic Melt: Molecular Dynamics Computer Simulation of Undercooled Al80Ni20,” Phys. Rev. B: Condens. Matter Mater. Phys. 78, 064208 (2008).
S. Gialanella and L. Lutterotti, Nanocrystalline Metallic Materials, Nanoclusters and Nanocrystals, Ed. by H. S. Nalwa (American Scientific Publisher, California, 2003), p. 1.
Y. Q. Cheng and E. Ma, “Atomic-Level Structure and Structure-Property Relationship in Metallic Glasses,” Progr. Mater. Sci. 56, 379–473 (2011).
J. Noro, A. S. Ramos, and M. T. Vieira, Intermetallic Phase Formation in Nanometric Ni/Al Multilayer Thin Films,” Intermetallics 16, 1061–1065 (2008).
G. Voronoi, “Recherches sur les paralléloèdres Primitives,” J. Reine Angew. Math. 134, 198–287 (1908).
B. J. Gellatly and J. L. Finney, “Characterisation of Models of Multicomponent Amorphous Metals: The Radical Alternative to the Voronoi Polyhedron,” J. Non-Cryst. Solids 50, 313–329 (1982).
M. Barth, B. Wei, and D. M. Herlach, “Crystal Growth in Undercooled Melts of the Intermetallic Compounds FeSi and CoSi,” Phys. Rev. B: Condens. Matter 51, 3422–3428 (1995).
K. Biswas G. Phanikumar, D. Holland-Moritz, D. M. Herlach, and K. Chattopadhyay, “Disorder Trapping and Grain Refinement during Solidification of Undercooled Fe-18 at % Ge Melts,” Phil. Mag. 87, 3817–3837 (2007).
H. Reichert, O. Klein, H. Dosch, M. Denk, V. Honkimaki, T. Lippmann, and G. Reiter, “Observation of Five-Fold Local Symmetry in Liquid Lead,” Nature 408, 839–841 (2000).
T. Schenk, D. Holland-Moritz, V. Simonet, R. Bellissent, and D. M. Herlach, “Icosahedral Short-Range Order in Deeply Undercooled Metallic Melts,” Phys. Rev. Lett. 89, 075507 (2002).
D. Holland-Moritz, T. Schenk, R. Bellissent, V. Simonet, K. Funakoshi, J. M. Merino, T. Buslaps, and S. Reutzel, “Short-Range Order in Undercooled Co Melts,” J. Non-Cryst. Solids 312–314, 47–51 (2002).
A. Di Cicco, A. Trapananti, S. Faggioni, and A. Filipponi, “Is There Icosahedral Ordering in Liquid and Undercooled Metals?” Phys. Rev. Lett. 91, 135505 (2003).
N. Jakse and A. Pasturel, “Local Order of Liquid and Supercooled Zirconium by Ab Initio Molecular Dynamics,” Phys. Rev. Lett. 91, 195501 (2003).
A. V. Evteev, A. T. Kosilov, and E. V. Levchenko, “Atomic Mechanisms of Pure Iron Vitrification,” J. Exp. Theor. Phys. 99, 522–529 (2004).
N. Jakse and A. Pasturel, “Ab Initio Molecular Dynamics Simulations of Local Structure of Supercooled Ni,” J. Chem. Phys. 120, 6124–6127 (2004).
P. Ganesh and M. Widom, “Signature of Nearly Icosahedral Structures in Liquid and Supercooled Liquid Copper,” Phys. Rev. B: Condens. Matter Mater. Phys. 74, 134205 (2006).
M. Celino, V. Rosato, A. Di Cicco, A. Trapananti, and C. Massobrio, “Role of Defective Icosahedra in Undercooled Copper.” Phys. Rev. B: Condens. Matter Mater. Phys. 75, 174210 (2007).
P. Ganesh and M. Widom, “Ab Initio Simulations of Geometrical Frustration in Supercooled Liquid Fe and Fe-Based Metallic Glass,” Phys. Rev. B: Condens. Matter Mater. Phys. 77, 014205 (2008).
H. Senapati, R. K. Kadiyala, and C. A. Angell, “Single- and Two-Step Calorimetric Studies of Crystallization Kinetics in Simple Ionic Glass-Forming Lliquids. 1. Ca(NO3)2-KNO3 System,” J. Phys. Chem. 95, 7050–7054 (1991).
S. Mukherjee, J. Schroers, W. L. Johnson, and W. K. Rhim, “Influence of Kinetic and Thermodynamic Factors on the Glass-Forming Ability of Zirconium-Based Bulk Amorphous Alloys,” Phys. Rev. Lett. 94, 245501 (2005).
R. Busch, J. Schroers, and W. H. Wang, “Thermodynamics and Kinetics of Bulk Metallic Glass,” MRS Bull. 32, 620–623 (2007).
A. V. Evteev, A. T. Kosilov, E. V. Levchenko, and O. B. Logachev, “The Influence of the Icosahedral Percolation Transition in Supercooled Liquid Iron on the Diffusion Mobility of Atoms,” J. Exp. Theor. Phys. 101, 521–527 (2005).
A. V. Evteev, A. T. Kosilov, E. V. Levchenko, and O. B. Logachev, “Kinetics of Isothermal Nucleation in a Supercooled Iron Melt,” Phys. Solid State 48, 815–820 (2006).
Y. Zhang, L. Wang, W. Wang, “Thermodynamic, Dynamic, and Structural Relaxation in Supercooled Liquid and Glassy Ni below the Critical Temperature,” J. Phys.: Condens. Matter. 19, 196106 (2007).
T. Mizuguchi and T. Odagaki, “Vitrification of a Monatomic 2D Simple Liquid,” Cent. Eur. J. Phys. 7, 479–482 (2009).
T. Mizuguchi and T. Odagaki, “Glass Formation and Crystallization of a Simple Monoatomic Liquid,” Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 79, 051501 (2009).
S.-H. Lee, J.-H. Lee, Y.-H. Lee, D. H. Shin, and Y. S. Kim, “Effect of Heating Rate on the Combustion Synthesis of Intermetallics,” Mater. Sci. Eng. A 281, 275–285 (2000).
K. Morsi, “Review: Reaction Synthesis Processing of Ni-Al Intermetallic Materials,” Mater. Sci. Eng. A 299, 1–15 (2001).
E. K. Y. Fu, R. D. Rawlings, and H. B. McShane, “Reaction Synthesis of Titanium Aluminides,” J. Mater. Sci. 36, 5537–5542 (2001).
N. Bertolino, M. Monagheddu, A. Tacca, P. Giuliani, C. Zanotti, and U. A. Tamburini, “Ignition Mechanism in Combustion Synthesis of Ti-Al and Ti-Ni Systems,” Intermetallics 11, 41–49 (2003).
M. Adeli, S. H. Seyedein, M. R. Aboutalebi, M. Kobashi, and N. Kanetake, “A Study on the Combustion Synthesis of Titanium Aluminide in the Self-Propagating Mode,” J. Alloys Compd. 497, 100–104 (2010).
L. Farber, L. Klinger, and I. Gotman, “Modeling of Reactive Synthesis in Consolidated Blends of Fine Ni and Al Powders,” Mater. Sci. Eng. A 254, 155–165 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Evteev, A.V., Levchenko, E.V., Belova, I.V. et al. Molecular dynamics simulation of surface segregation, diffusion and reaction phenomena in equiatomic Ni-Al systems. Phys. Metals Metallogr. 113, 1202–1243 (2012). https://doi.org/10.1134/S0031918X12130017
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X12130017