Abstract
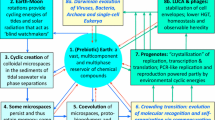
Evolution of the prokaryotic biosphere is regarded from the system point of view. It starts with the appearance of the first organisms, the ∼3.5 Ga date forming the boundary between the observed and imagined biosphere. The prokaryotic community dominated from the Archean to the Mesoproterozoic. Prokaryotes make a sustainable community due to the cooperative action of specialized forms. The main route for establishing a community is made by trophic links. The structure of the trophic links in the prokaryotic community making a trophic network is an invariant, with secondary adaptive deviations. Material balance is the ultimate requirement for a long living self-supporting system. The system of biogeospheric cycles is dictated by the constancy of biomass composition establishing a quantitative ratio between Corg:Norg:Porg. Biospheric processes are driven by the Corg-cycle. Carbon assimilation is limited by the size of the illuminated moist surface populated by producers, meaning that Corg-production remains within an order of magnitude of 102 Gt/yr. Evolution of primary producers forms a basis for the evolution of the biospheric-geospheric system, and cyanobacteria integrated as chloroplasts remain its driving force. Decomposition of organic compounds is performed by organotrophic destructors, anacrobic being less effective. Destructors determine the residual Corg accumulation. Recalcitrant Corg remaining in the sedimentary record is equilibrated by O2 and other oxidized compounds as Fe-oxides or sulfates. Geospheric and biotic interactions include both direct and biotically mediated processes; the most important is the weathering-sedimentation pathway. Prokaryotic community makes a sustainable frame into which all other more complex forms of life fit. That makes the prokaryotic biosphere a permanent essence of the whole system. New participants might come in and substitute functional components only when they fit to the existing system. The evolution of a large system is additive rather than substitutive. The message of this is; “we all originated from the cyanobacterial community.”
Similar content being viewed by others
References
G. Arp, “Calcification of Non-marine Cyanobacterial Biofilms (USA, PR China, Indonesia, Germany)—Implications for the Interpretation of Fossil Microbialites,” Dissertation Georg-August-Universität Göttingen (1999) [delivered by the courtesy of the author].
G. Arp, A. Reimer, and J. Reitner, “Calcification in Cyanobacterial Biofilms of Alkaline Salt Lakes,” Eur. J. Phycol. 34, 393–403 (1999).
E. A. Bonch-Osmolovskaya, M. L. Miroshnichenko, T. G. Sokolova, and A. I. Slobodkin, “Thermophilic Microbial Communities: New Physiological Groups, New Habitats,”, Tr. Winogradsky Inst. Microbiol. 12, 29–40 (2004).
T. D. Brock, Thermophiles: General, Molecular, and Applied Microbiology (Wiley & Sons, New York, 1986).
Y. Cohen and E. Rosenberg (Eds.) Microbial Mats: Physiological Ecology of Benthic Microbial Communities (ASM, Washington DC, 1989).
S. A. Franck and G. A. Zavarzin, “What Are the Necessary Conditions for Origin of Life and Subsequent Planetary Life-Support Systems?,” in Dahlem Konferenzien 91: Earth System Analysis for Sustainability (MIT Press, Cambridge Massachusetts, London UK, 2003), pp. 74–90.
M. Heidegger, Was heißt Denken? Vorlesung Wintersemestr 1951/52 (Philipp Reclam jun., Stuttgart, 1984).
L. M. Gerasimenko and V. K. Orleansky, “Actualistic Paleontology of Cyanobacteria,” Tr. Winogradsky Inst. Microbiol. 12, 80–108 (2004).
L. M. Gerasimenko and G. A. Zavarzin, “Metabolism of H2 in Cyanobacterial Communities,” Microbiologia 51, 718–722 (1982).
J. P. Grotzinger, “Facies and Evolution of Precambrian Carbonate Depositional Systems: Emergence of the Modern Platform Archetype, Controls on Carbonate Platform and Basin Development,” SEPM Spec. Publ. 44, 79–104 (1989).
G. A. Karpov, V. A. Eroschev-Shakh, and G. A. Zavarzin, “Role of Biogenic Factor in the Formation of Environment for the Zone of Argillization in the Contemporary Hydrothermal Systems and Solfataric Fields,” Vulkanol. Seismol. 2, 64–74 (1984).
J. D. Keasling, S. J. van Dien, P. Trelstaad, et al., “Application of Polyphosphate Metabolism to Environmental and Biotechnological Problems,” Biokhimia 65, 394–404 (2000) (and other papers in this special issue).
A. H. Knoll, Life on a Young Planet (Princeton Univ. Press., 2003).
W. E. Krumbein, D. M. Paterson, and L. J. Stal (Eds.), Biostabilization of Sediments: Microbially Mediated Processes in Tide Influenced Deposits and Their Importance in Stabilization and Diagenesis of Sediments (Oldenburg, Universität, 1994).
W. E. Krumbein, D. M. Paterson, and G. A. Zavarzin (Eds.), Fossil and Recent Biofilms: A Natural History of Life on Earth (Kluwer Acad., Dordrecht, 2003).
E. V. Kupriyanova, A. G. Markelova, and N. V. Lebedeva, “Carbonic Anhydrase of the Alkaliphilic Cyanobacterium Microcoleus chthonoplastes,” Microbiology 73, 307–311 (2004).
V. A. Melezhik, A. E. Fallick, V. V. Makarikhin, and V. V. Lyubtsov, “Links between Paleoproterozoic Paleogeography and Rise and Decline of Stromatolites: Fennoscandian Shield,” Precambr. Res. 82, 311–348 (1997).
V. A. Melezhik, A. E. Fallick, P. V. Medvedev, and V. V. Makarikhin, “Paleoproterozoic Magnesite: Lithological and Isotopic Evidence for Playa/Sabkha Environments,” Sedimentology 48, 379–397 (2001).
V. A. Melezhik, A. E. Fallick, D. V. Rychanchik, and A. B. Kuznetzov, “Palaeoproterozoik Evaporates in Fennoscandia: Implications for Seawater Sulphate, the Rise of Atmospheric Oxygen and Local Amplification of the σ13C Excursion,” Terra Nova 17, 141–148 (2005).
M. L. Miroshnichenko, “Thermophilic Microbial Communities of Deep-sea Hydrotherms, Review,” Mikrobiologiya 73, 5–18 (2004).
V. R. Phoenix, K. O. Konhauser, D. G. Adams, and S. H. Botrell, “Role of Biomineralzation As an Ultraviolet Shield: Implications for Achaean Life,” Geology 29(9), 823–826 (2001).
G. J. Retallack, Soils of the Past: An Introduction to Paleopedology (Unwyn Hyman, Boston, 1990).
M. Schidlowski, “Sedimentary Carbon Isotope Archives As Recorders of Early Life: Implications for Extraterrestrial Scenarios,” in Fundamentals of Life (Elsevier, 2002), pp. 308–329.
D. W. Schwartzman, Life, Temperature, and the Earth: The Self-organizing Biosphere (Columbia Univ. Press, New York, 1999).
V. N. Sergeev, L. M. Gerasimenko, and G. A. Zavarzin, “Proterozoic History and Present State of Cyanobacteria,” Mikrobiologiya 71(6), 725–40 (2002).
M. Sharma and V. N. Sergeev, “Genesis of Carbonate Precipitate Patterns and Associated Microfossils in Mesoproterozoic Formations of India and Russia—A Comparative Study,” Precambr. Res. 134, 317–347 (2004).
A. I. Slobodkin, V. A. Eroschew-Shakh, N. A. Kostrikina, et al., “The Formation of Magnetite by Thermophilic Anaerobic Microorganisms,” Dokl. Akad. Nauk 345, 694–697 (1995).
L. J. Stal and P. Caumette (Eds.), “Microbial Mats: Structure, Development and Environmental Significance,” NATO ASI G 35, 443–452 (1994).
V. A. Svetlichny, T. G. Sokolova, M. Gerchardt, et al., “Carboxydothermus hydrogenoformans gen. nov., sp. nov. a New CO-utilizing Thermophilic Anaerobic Bacterium from Hydrothermal Environments of Kunashir Island,” System. Appl. Microbiol. 14, 254–260 (1991).
F. Westall and F. D. Drake, “Is Life Unavoidable Planetary Phenomenon Given the Right Conditions?,” in Dahlem Koferenzien 91: Earth System Analysis for Sustainability (MIT Press Cambridge Massachusets, London UK, 2004), pp. 53–72.
F. Westall, M. J. de Witt, J. de Ronde, and D. Gerneke, “Early Archean Fossil Bacteria and Biofilms in Hydrothermally-influenced Sediments from the Barberton Greenstone Belt, South Africa,” Precambr. Res. 106, 93–116 (2001).
W. D. Whitman, D. C. Coleman, and W. J. Wiebe, “Prokaryotes: The Unseen Majority,” Proc. Nat. Acad. Sci. 95(12), 6578–6583 (1998).
C. R. Woese, “A New Biology for a New Century,” Microbiol. Molec. Biol. Rev. 68(2), 173–186 (2004).
G. A. Zavarzin, Bacteria and the Composition of the Atmosphere (Nauka, Moscow, 1984) [in Russian].
G. A. Zavarzin, “Epicontinental Soda Lakes As Supposed Relict Biotopes for the Formation of Terrestrial Biota, Mikrobiologiya 62(5), 789–800 (1993).
G. A. Zavarzin, “Diversity of Cyanobacterial Mats,” in Fossil and Recent Biofilms: A Natural History of Life on Earth (Kluwer Acad., Dordrecht, 2003), pp. 141–150.
G. A. Zavarzin, “Coming-into-Being of the System of Biogeochemical Cycles,” Paleontol. J., No. 6, 16–24 (2003).
G. A. Zavarzin, Lectures in Environmental Microbiology (Nauka, Moscow, 2003) [in Russian].
G. A. Zavarzin, L. M. Gerasimenko, and T. N. Zhilina, “Cyanobacterial Communities in Hypersaline Lagoons of Lake Sivash,” Mikrobiologiya 62(6), 579–599 (1993).
G. A. Zavarzin, G. A. Karpov, V. M. Gorlenko, et al., Calderic Microorganisms (Nauka, Moscow, 1989) [in Russian].
G. A. Zavarzin and T. N. Zhilina, “Anaerobic Chemotrophic Alkaliphiles,” in Jurney to Diverse Microbial Worlds (Kluwer Acad., The Netherlands, 2000), pp. 191–208.
G. A. Zavarzin, T. N. Zhilina, and V. V. Kevbrin, “The Alkaliphilic Microbial Community and Its Functional Diversity,” Mikrobiologiya 68(5), 503–521 (1999).
D. G. Zavarzina, “The Formation of Magnetite and Siderite by Thermophilic Fe(III)-Reducing Bacteria,” Paleontol. J. 38(6), 585–589 (2004).
Author information
Authors and Affiliations
Additional information
The text was submitted by the author in English.
Rights and permissions
About this article
Cite this article
Zavarzin, G.A. Microbial biopshere. Paleontol. J. 40, S425–S433 (2006). https://doi.org/10.1134/S0031030106100029
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0031030106100029