Abstract
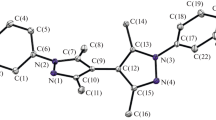
The [Rh(Hdp)2(N∧N)]ClO4 complexes (Hdp− is the monodeprotonated form of 4,6-diphenylpyrimidine and (N∧N) is ethylenediamine, 2,2′-bipyridyl, and 1,10-phenanthroline) are synthesized and characterized by 1H and 13C NMR, IR, electronic absorption, and emission spectroscopy, as well as by cyclic voltammetry. The magnetic equivalence of two cyclometalated 4,6-diphenylpyrimidine ligands in the composition of complexes points to the cis position of metalated phenyl rings in the inner sphere. Quasi-reversible one-electron reduction waves are attributed to the ligand-centered electron transfer to the π* antibonding orbital of heterocyclic ligands, while irreversible oxidation waves are associated with electron detachment from the Rh-C σ bonding orbital of the {Rh(Hdp)2} metal-complex fragment. The characteristic long-wave-length absorption bands and the vibrationally structured phosphorescence bands of complexes are assigned to the spin-allowed and spin-forbidden charge-transfer optical transitions between the σRh-C and πHdp* orbitals localized on the {Rh(Hdp)2} fragment of the complex.
Similar content being viewed by others
References
G-J. Zhou, X-Z. Wang, W-Y. Wong, et al., J. Organomet. Chem. 692(16), 3461 (2007).
S-Y. Kim, J-H. Kim, Y. Ha, et al., Curr. Appl. Phys. 7(4), 380 (2007).
A. B. Tamaoy, S. Garon, T. Sajoto, et al., Inorg. Chem. 44(24), 8723 (2005).
S. Lamanski, P. Djurovich, D. Murphy, et al., J. Am. Chem. Soc. 123(18), 4304 (2001).
N. Agarwal and P. K. Nayak, Tetrahedron Lett. 49(17), 2710 (2008).
E. V. Ivanova, M. V. Puzyk, and K. P. Balashev, Zh. Org. Khim. 78(6), 1008 (2008).
E. V. Ivanova, M. V. Puzyk, and K. P. Balashev, Opt. Spektrosk. 106(3), 409 (2009).
V. V. Vasil’ev, K. P. Balashev, and G. A. Shagisultanova, Opt. Spektrosk. 54, 876 (1983).
V. S. Kotlyar and K. P. Balashev, Elektrokhimiya 32(11), 1358 (1996).
H. Brederick, R. Gompper, and G. Morlock, Chem. Ber. 90(6), 942 (1957).
S. Sprouse, K. A. King, P. J. Spellane, and R. J. Watts, J. Am. Chem. Soc. 106(22), 6647 (1984).
K. Nakanishi, Infrared Absorption Spectroscopy (Holden-Day, San Francisco, 1962; Mir, Moscow, 1965).
G. B. Caygill, R. M. Hartsorn, and P. J. Stell, J. Organomet. Chem. 382(3), 455 (1990).
M. K. DeArmond, K. W. Hanck, and D. W. Wertz, Coord. Chem. Rev. 64, 65 (1985).
T. Koopmans, Physics 1(1), 104 (1933).
P. Didier and I. Ortmans, Inorg. Chem. 32(23), 5239 (1993).
I. Ortmans and P. Didier, Inorg. Chem. 34(14), 3695 (1995).
Y. Ohsawa, S. Sprouse, K. A. King, et al., J. Phys. Chem. 91(5), 1047 (1987).
A. A. Vlcek, Coord. Chem. Rev. 43, 39 (1982).
D. Sandrini, M. Maestry, V. Balzani, et al., Inorg. Chem. 27(15), 2640 (1988).
M. Maestry, D. Sandrini, V. Balzani, et al., Inorg. Chem. 26(8), 1323 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Ivanova, K.P. Balashev, 2010, published in Optika i Spektroskopiya, 2010, Vol. 108, No. 4, pp. 611–617.
Rights and permissions
About this article
Cite this article
Ivanova, E.V., Balashev, K.P. Optical and electrochemical properties of cyclometalated Rh(III) complexes based on 4,6-diphenylpyrimidine with ethylenediamine, 2,2′-bipyridyl, and 1,10-phenanthroline. Opt. Spectrosc. 108, 574–580 (2010). https://doi.org/10.1134/S0030400X10040119
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X10040119