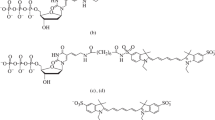
Abstract
A genotyping procedure based on single-step PCR and subsequent allele-specific hybridization on a hydrogel biochip was developed to address the polymorphisms of HERC2, OCA2, SLC24A4, SLC45A2, TYR, IRF4, MC1R, MITF, PIGU, MYH7B, NCOA6, and CDK10. Amplified gene fragments were fluorescently labeled in PCR, and fluorescent signals from biochip cells were detected to evaluate how efficiently the PCR product formed a perfect duplex with an immobilized probe. The analytical characteristics of hybridization analysis were estimated for several fluorophores with different optical spectra. Cyanine dyes fluorescing in the range of Cy5 and Cy7 were synthesized for the purpose and used as 5'-tags of universal primers in single-step PCR. A Cy7 analog fluorescing in the near infrared range was found to increase the sensitivity of hybridization analysis by producing a lower background signal in the cases where target gene amplification was low.


Similar content being viewed by others
REFERENCES
Chernenko P.A., Peterson S.B., Lyubchenko L.N. 2012. Hereditary cutaneous melanoma: Clinical–molecular diagnosis. Ross. Bioterapevt. Zh. 11 (3), 81–88.
Nan H., Kraft P., Hunter D.J., Han J. 2009. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int. J. Cancer. 125, 909–917.
Ferguson R., Vogelsang M., Ucisik-Akkaya E., Rai K., Pilarski R., Martinez C.N., Rendleman J., Kazlow E., Nagdimov K., Osman I., Klein R.J., Davidorf F.H., Cebulla C.M., Abdel-Rahman M.H., Kirchhoff T. 2016. Genetic markers of pigmentation are novel risk loci for uveal melanoma. Sci. Rep. 6, 31191.
Stefanaki I., Panagiotou O.A., Kodela E., Gogas H., Kypreou K.P., Chatzinasiou F., Nikolaou V., Plaka M., Kalfa I., Antoniou C., Ioannidis J.P., Evangelou E., Stratigos A.J. 2013. Replication and predictive value of SNPs associated with melanoma and pigmentation traits in a Southern European case-control study. PLoS One. 8, e55712.
Ibarrola-Villava M., Kumar R., Nagore E., Benfodda M., Guedj M., Gazal S., Hu H.H., Guan J., Rachkonda P.S., Descamps V., Basset-Seguin N., Bensussan A., Bagot M., Saiag P., Schadendorf D., et al. 2015. Genes involved in the WNT and vesicular trafficking pathways are associated with melanoma predisposition. Int. J. Cancer. 136, 2109–2119.
Kosiniak-Kamysz A., Marczakiewicz-Lustig A., Marcińska M., Skowron M., Wojas-Pelc A., Pośpiech E., Branicki W. 2014. Increased risk of developing cutaneous malignant melanoma is associated with variation in pigmentation genes and VDR, and may involve epistatic effects. Melanoma Res. 24, 388–396.
Duffy D.L., Zhao Z.Z., Sturm R.A., Hayward N.K., Martin N.G., Montgomery G.W. 2010. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J. Invest. Dermatol. 130, 520–528.
Helsing P., Nymoen D.A., Rootwelt H., Vårdal M., Akslen L.A., Molven A., Andresen P.A. 2012. MC1R, ASIP, TYR, and TYRP1 gene variants in a population-based series of multiple primary melanomas. Genes Chromosomes Cancer. 51, 654–661.
Yokoyama S., Woods S.L., Boyle G.M., Aoude L.G., MacGregor S., Zismann V., Gartside M., Cust A.E., Haq R., Harland M., Taylor J.C., Duffy D.L., Holohan K., Dutton-Regester K., Palmer J.M., et al. 2011. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature. 480, 99–103.
Mangas C., Potrony M., Mainetti C., Bianchi E., Carrozza Merlani P., Mancarella Eberhardt A., Maspoli-Postizzi E., Marazza G., Marcollo-Pini A., Pelloni F., Sessa C., Simona B., Puig-Butillé J.A., Badenas C., Puig S. 2016. Genetic susceptibility to cutaneous melanoma in southern Switzerland: Role of CDKN2A, MC1R and MITF. Br. J. Dermatol. 175, 1030–1037.
Gromowski T., Masojć B., Scott R.J., Cybulski C., Górski B., Kluźniak W., Paszkowska-Szczur K., Rozmiarek A., Dębniak B., Maleszka R., Kładny J., Lubiński J., Dębniak T. 2014. Prevalence of the E318K and V320I MITF germline mutations in Polish cancer patients and multiorgan cancer risk: A population-based study. Cancer Genet. 207, 128–132.
Debniak T., Gapska P., Serrano-Fernandez P., Rassoud I., Cybulski C., Maleszka R., Sulikowski M., Narod S., Lubiński J. 2010. Modest association of malignant melanoma with the rs910873 and rs1885120 markers on chromosome 20: A population-based study. Melanoma Res. 20, 159–160.
Maccioni L., Rachakonda P.S., Scherer D., Bermejo J.L., Planelles D., Requena C., Hemminki K., Nagore E., Kumar R. 2013. Variants at chromosome 20 (ASIP locus) and melanoma risk. Int. J. Cancer. 132, 42–54.
Fesenko D.O., Kalennik O.V., Barsky V.E., Zasedatelev A.S., Nasedkina T.V. 2012. Biochip development for determining Y-haplogroups that occur in Russian populations. Mol. Biol. (Moscow). 46 (5), 731–734.
Fesenko D.O., Mityaeva O.N., Nasedkina T.V., Rubtsov P.M., Lysov Yu.P., Zasedatelev A.S. 2010. HLA-DQA1, AB0, and AMEL genotyping of biological material with biochips. Mol. Biol. (Moscow). 44 (3), 401–406.
Fesenko D.O., Chudinov A.V., Surzhikov S.A., Nasedkina T.V., Zasedatelev A.S. 2014. Biochip for genotyping SNPs defining core Y-chromosome haplogroups in Russian population groups. BioChip J. 8, 171–178.
Spitsyn M.A., Shershov V.E., Kuznetsova V.E., Barsky V.E., Egorov E.E., Emelyanova M.A., Kreindlin E.Ya., Lysov Yu.P., Guseinov T.O., Fesenko D.E., Lapa S.A., Surzhikov S.A., Abramov I.S., Nasedkina T.V., Zasedatelev A.S., Chudinov A.V. 2015. Infrared fluorescent markers for microarray DNA analysis. Mol. Biol. (Moscow). 49 (5), 7678–686.
Fesenko D.O., Chudinov A.V., Surzhikov S.A., Zasedatelev A.S. 2016. Biochip-based genotyping assay for detection of polymorphisms in pigmentation genes associated with cutaneous melanoma. Genet. Test. Mol. Biomarkers. 20, 208–212.
Boyer A.E., Lipowska M., Zen J.M., Patonay G. 1992. Evaluation of near infrared dyes as labels for immunoassays utilizing laser diode detection: Development of near infrared dye immunoassay (NIRDIA). Anal. Lett. 25, 415–428.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Tkacheva
Abbreviations: CDK4, cyclin-dependent kinase 4 gene; CDK10, cell division protein kinase 10 gene; CDKN2A, cyclin-dependent kinase inhibitor 2A gene; GWAS, genome-wide association study; HERC2, HECT domain and RCC1-like domain 2 gene; IRF4, interferon regulatory factor 4 gene; MC1R, melanocortin 1 receptor gene; MITF, microphthalmia-associated transcription factor gene; MYH7B, myosin heavy chain 7B gene; NCOA6, nuclear receptor coactivator 6 gene; OCA2, oculocutaneous albinism II gene; PIGU, phosphatidylinositol glycan anchor biosynthesis, class U protein gene; PTEN, phosphatase and tensin homolog gene; SLC24A4, solute carrier family 24 member 4 gene; SLC45A2, solute carrier family 45 member 2 gene; TP53, tumor protein 53 (p53) gene; TYR, tyrosinase gene.
Rights and permissions
About this article
Cite this article
Fesenko, D.O., Abramov, I.S., Shershov, V.E. et al. Multiplex Assay to Evaluate the Genetic Risk of Developing Human Melanoma. Mol Biol 52, 865–871 (2018). https://doi.org/10.1134/S0026893318060079
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893318060079