Abstract
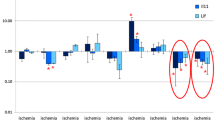
Neurotrophins regulate key functions of nervous tissue cells. Analysis of neurotrophin mRNA expression is an appropriate tool to assess therapeutic efficiency of antistroke drugs. We have analyzed the effect of synthetic peptide semax and its C-terminal Pro-Gly-Pro tripeptide on mRNA expression of neurotrophins Ngf, Bdnf, and Nt-3 and their receptors TrkA, TrkB, TrkC, and p75 in rat frontal cortex, hippocampus, and cerebellum after bilateral common carotid artery occlusion. The animals were decapitated at 30 min and 1, 2, 4, 8, 12, and 24 h after the operation. The mRNA expression of neurotrophins and their receptors was assessed by relative quantification using real-time RT-PCR. Our results demonstrated that ischemia caused a significant decrease in gene expression in the hippocampus. Semax and PGP treatment affected the expression of neurotrophins and their receptors predominantly in the frontal cortex and hippocampus of the ischemized animals. In the frontal cortex, Semax treatment resulted in a decrease of mRNA level of neurotrophin receptors, while PGP treatment increased the level of these mRNA. Maximal neuroprotective effect of both peptides was observed in the hippocampus 12 h after occlusion. A decrease of gene expression of neurotrophins and their receptors caused by the occlusion was overcome by Semax and PGP. These results clarify the mechanism of Semax action and reveal certain features of mRNA expression of neurotrophins and their receptors under experimental conditions.
Similar content being viewed by others
Abbreviations
- ACTH:
-
adrenocorticotropic hormone
- PCR:
-
polymerase chain reaction
- RT:
-
reverse transcription
- GAPDH:
-
glyceraldehyde 3-phosphate dehydrogenase
References
Huang E.J., Reichardt L.F. 2001. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736.
Huang E.J., Reichardt L.F. 2003. Trk receptors: Roles in neuronal signal transduction. Annu. Rev Biochem. 72, 609–642.
Kim M.W., Bang M.S., Han T.R., Ko Y.J., Yoon B.W., Kim J.H., Kang L.M., Lee K.M., Kim M.H. 2005. Exercise increased BDNF and trkB in the contralateral hemisphere of the ischemic rat brain. Brain Res. 1052, 16–21.
Wu D. 2005. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx. 2, 120–128.
Zhang Z.H., Wang R.Z., Wang R.Z., Li G.L., Wei J.J., Li Z.J., Feng M., Kang J., Du W.C., Ma W.B., Li Y.N., Yang Y., Kong Y.G. 2008. Transplantation of neural stem cells modified by human neurotrophin-3 promotes functional recovery after transient focal cerebral ischemia in rats. Neurosci. Lett. 444, 227–230.
Gusev E.I., Skvortsova V.I. 2001. Ishemiya golovnogo mozga (Cerebral Ischemia). Moscow: Meditsina.
Shadrina M.I., Dolotov O.V., Grivennikov I.A., Slominsky P.A., Andreeva L.A., Inozemtseva L.S., Limborska S.A., Myasoedov N.F. 2001. Rapid induction of neurotrophin mRNAs in rat glial cell cultures by Semax, an adrenocorticotropic hormone analog. Neurosci. Lett. 308, 115–118.
Dolotov O.V., Karpenko E.A., Inozemtseva L.S., Seredenina T.S., Levitskaya N.G., Rozyczka J., Dubynina E.V., Novosadova E.V., Andreeva L.A., Alfeeva L.Y., Kamensky A.A., Grivennikov I.A., Myasoedov N.F., Engele J. 2006. Semax, an analog of ACTH(4-10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus. Brain Res. 1117, 54–60.
Agapova T.Y., Agniullin Y.V., Shadrina M.I., Shram S.I., Slominsky P.A., Lymborska S.A., Myasoedov N.F. 2007. Neurotrophin gene expression in rat brain under the action of Semax, an analogue of ACTH 4–10. Neurosci. Lett. 417, 201–205.
Shadrina M., Kolomin T., Agapova T., Agniullin Y., Shram S., Slominsky P., Lymborska S., Myasoedov N. 2010. Comparison of the temporary dynamics of NGF and BDNF gene expression in rat hippocampus, frontal cortex, and retina under Semax action. J. Mol. Neurosci. 41, 30–35.
Martynova K.V., Andreeva L.A., Klimova P.A., Kirillova Yu.G., Shevchenko V.P., Nagaev I.Yu., Shram S.I., Shvets V.I., Myasoedov N.F 2009. Structural-functional study of glycine-and-proline-containing peptides (glyprolines) as potential neuroprotectors. Russ. J. Bioorg. Chem. 35, 150–156.
Storozhevykh T.P., Tukhbatova G.R., Senilova Ya.E., Pinelis V.G., Andreeva L.A., Myasoedov N.F. 2007. Effect of Semax and its Pro-Gly-Pro fragment on the calcium homeostasis of neurons and their survival under conditions of glutamate toxicity. Bull. Exp. Biol. Med. 143, 501–604.
Astashkin E.I., Bespalova Yu.B., Grivennikov I.A., Smirnov O.N., Glezer M.G., Myasoedov N.F. 2000. Effects of Semax on Ca2+ responses of human neutrophils. Doklady Biol. Sci. 374, 536–538.
Bashkatova V.G., Koshelev V.B., Fadyukova O.E., Alexeev A.A., Vanin A.F., Rayevsky K.S., Ashmarin I.P., Armstrong D.M. 2001. Novel synthetic analogue of ACTH 4-10 (Semax) but not glycine prevents the enhanced nitric oxide generation in cerebral cortex of rats with incomplete global ischemia. Brain Res. 894, 145–149.
Stavchansky V.V., Yuzhakov V.V., Botsina A.Y., Skvortsova V.I., Bondurko L.N., Tsyganova M.G., Limborska S.A., Myasoedov N.F., Dergunova L.V. 2010. The effect of Semax and its C-end peptide PGP on the morphology and proliferative activity of rat brain cells during experimental ischemia: A pilot study. J. Mol. Neurosci. [epub ahead of print]
Grivennikov I.A., Dolotov O.V., Gol’dina Yu.I. 1999. Peptide factors in proliferation, differentiation, and viability of the cells of mammalian nervous system. Mol. Biol. (Moscow). 33, 103–108.
Chomczynski P., Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal. Biochem. 162, 156–159.
Maniatis, T., Fritsch, E.F., Sambrook, J. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press.
Medhurst A.D., Harrison D.C., Read S.J., Campbell C.A., Robbins M.J., Pangalos M.N. 2000. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J. Neurosci. Methods. 98, 9–20.
Harrison D.C., Medhurst A.D., Bond B.C., Campbell C.A., Davis R.P., Philpott K.L. 2000. The use of quantitative RT-PCR to measure mRNA expression in a rat model of focal ischemia-caspase-3 as a case study. Mol. Brain. Res. 75, 143–149.
Pfaffl M.W. 2010. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45.
Pfaffl M.W., Horgan G.W., Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30, e36.
Kokaia Z., Andsberg G., Yan Q., Lindvall O. 1998. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia:evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 154, 289–301.
Lee T.H., Kato H., Chen S.T., Kogure K., Itoyama Y. 2002. Expression disparity of brain-derived neurotrophic factor immunoreactivity and mRNA in ischemic hippocampal neurons. NeuroReports. 13, 2271–2275.
Higuchi T., Graham S.H., Fernandez E.J., Rooney W.D., Gaspary H.L., Weiner M.W., Maudsley A.A. 1997. Effects of severe global ischemia on N-acetylaspartate and other metabolites in the rat brain. Magn. Reson. Med. 37, 851–857.
Sieber F.E., Palmon S.C., Traystman R.J., Martin L.J. 1995. Global incomplete cerebral ischemia produces predominantly cortical neuronal injury. Stroke. 26, 2091–2096.
Almeida R.D., Manadas B.J., Melo C.V., Gomes J.R., Mendes C.S., Grãos M.M., Carvalho R.F., Carvalho A.P., Duarte C.B. 2005. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 12, 1329–1343.
Schäbitz W.R., Schwab S., Spranger M., Hacke W. 1997. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 17, 500–506.
Larsson E., Nanobashvili A., Kokaia Z., Lindvall O. 1999. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J. Cereb. Blood Flow Metab. 19, 1220–1228.
Zhang Y., Pardridge W.M. 2001. Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood-brain barrier drug targeting system. Stroke. 6, 1378–1384.
Li J.M., Brackmann D.E., Hitselberger W.E., Linthicum F.H. Jr, Lim D.J. 1999. Coexpression of neurotrophic growth factors and their receptors in human facial motor neurons. Ann. Otol. Rhinol. Laryngol. 108, 903–908.
Pitts A.F., Miller M.W. 2000. Expression of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 in the somatosensory cortex of the mature rat: Coexpression with high-affinity neurotrophin receptors. J. Comp. Neurol. 418, 241–254.
V’yunova T.V., Shevchenko K.V., Shevchenko V.P., Bobrov M.Yu., Bezuglov V.V., Myasoedov N.F. 2006. Specific binding opf Semax in different regions of the rat brain. Doklady Biol. Sci. 410, 376–377.
Dmitrieva V.G., Povarova O.V., Skvortsova V.I., Limborska S.A., Myasoedov N.F., Dergunova L.V. 2010. Semax and Pro-Gly-Pro activate the transcription of neurotrophins and their receptor genes after cerebral ischemia. Cell Mol. Neurobiol. 30, 71–79.
Yakovleva E.V., Kuzenkov V.S., Fedorov V.N., Skvortsova V.I., Koshelev V.B., Gusev E.I., Ashmarin I.P. 1999. In vivo efficiency of Semax in global cerebral ischemia. Bull. Exp. Biol. Med. 128, 806–807.
Coppola V., Barrick C.A., Southon E.A., Celeste A., Wang K., Chen B., Haddad el-B., Yin J., Nussenzweig A., Subramaniam A., Tessarollo L. 2004. Ablation of TrkA function in the immune system causes B cell abnormalities. Development. 131, 5185–5195.
Kermani P., Rafii D., Jin D.K., Whitlock P., Schaffer W., Chiang A., Vincent L., Friedrich M., Shido K., Hackett N.R., Crystal R.G., Rafii S., Hempstead B.L. 2005. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Invest. 115, 653–663.
Donovan M.J., Lin M.I., Wiegn P., Ringstedt T., Kraemer R., Hahn R., Wang S., Ibanez C., Rafii S., Hempstead B.L. 2000. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 127, 4531–4540.
Lin M.I., Das I., Schwartz G.M., Tsoulfas P., Mikawa T., Hempstead B.L. 2000. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev. Biol. 226, 180–191.
Dmitrieva V.G., Dergunova L.V., Povarova O.V., Skvortsova V.I., Limborska S.A., Myasoedov N.F. 2008. The effect of Semax and the C-terminal peptide PGP on expression of growth factor genes and receptors in rats under conditions of experimental cerebral ischemia. Doklady Biochem. Biophys. 422, 261–264.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Stavchansky, T.V. Tvorogova, A.Yu. Botsina, V.I. Skvortsova, S.A. Limborska, N.F. Myasoedov, L.V. Dergunova, 2011, published in Molekulyarnaya Biologiya, 2011, Vol. 45, No. 6, pp. 1026–1035.
Rights and permissions
About this article
Cite this article
Stavchansky, V.V., Tvorogova, T.V., Botsina, A.Y. et al. Effect of semax and its C-terminal peptide PGP on expression of neurotrophins and their receptors in rat brain during incomplete global ischemia. Mol Biol 45, 941–949 (2011). https://doi.org/10.1134/S0026893311050128
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893311050128