Abstract
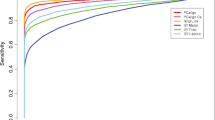
The results of testing the recognition ability of various amino acid substitution matrices and manifold (both extracted from the literature and of our own design) pseudopotentials intended for the recognition of protein structures and sequence-to-structure alignments are described. The numerical estimates of the recognition ability of various substitution matrices and pseudopotentials were obtained for different levels of protein structure similarity. It is demonstrated that substitution matrices work much better than pseudopotentials at a high degree of sequence similarity of spatially similar proteins; however, some pseudopotentials outdo substitution matrices at a low level of sequence similarity between analogous proteins.
Similar content being viewed by others
Abbreviations
- AA:
-
amino acid and 3D is three-dimensional
References
Kopp J., Bordoli L., Battey J.N.D., Kiefer F., Schwede T. 2007. Assesment of CASP7 predictions for templatebased modeling targets. Proteins. 69, S8, 38–56.
Dayhoff M.O., Schwartz R.M., Orcutt B.C. 1978. A model of evolutionary change in proteins. In: Atlas of Protein Sequence and Structure, vol. 5,suppl. 3. Ed. Dayhoff M.O. Washington, DC: Natl. Biomed. Res. Found., pp. 345–352.
Schwartz R.M., Dayhoff M.O. 1978. Matrices for detecting distant relationships. In: Atlas of Protein Sequence and Structure, vol. 5,suppl. 3. Ed. Dayhoff M.O. Washington, DC: Natl. Biomed. Res. Found., pp. 353–358.
Smith T.F., Waterman M.S. 1981. Identification of common molecular subsequences. J. Mol. Biol. 147, 195–197.
Altschul S.F., Gish W., Miller W., Myers E., Lipman D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410; http://www.ebi.ac.uk/blast.
Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. 1997. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402; http://www.ebi.ac.uk/psiblast.
Russell R.B., Copley R.R., Barton G.J. 1996. Protein fold recognition by mapping predicted secondary structures. J. Mol. Biol. 259, 349–365.
Wallqvist A., Fukunishi Y., Murphy L.R., Fadel A., Levy R.M. 2000. Iterative sequence/secondary structure search for protein homologs: Comparison with amino acid sequence alignments and application to fold recognition in genome databases. Bioinformatics. 16, 988–1002.
Litvinov I.I., Lobanov M.Yu., Mironov A.A., Finkelstein A.V., Roytberg M.A. 2006. Information on the secondary structure improves the quality of protein sequence alignment. Mol. Biol. 40, 533–540.
Finkelstein A.V., Reva B.A. 1991. Search for the most stable folds of protein chains. Nature. 351, 497–499.
Bowie J.U., Luthy R., Eisenberg D. 1991. A method to identify protein sequences that fold into a known threedimensional structure. Science. 253, 164–170.
Finkelstein A.V., Reva B.A. 1990. Globular protein threading by a self-consisted field method. 35, 402–406.
Miyazawa S., Jernigan R.L. 1985. Estimation of effective interresidue contact energies from protein crystal structures: Quasi-chemical approximation. Macromolecules. 18, 534–552.
Blundell T.L., Sibanda B.L., Sternberg M.J., Thornton J.M. 1987. Knowledge-based prediction of protein structures and the design of novel molecules. Nature. 326, 347–352.
Sippl M.J. 1990. Calculation of conformational ensembles from potentials of mean force: An approach to the knowledge-based prediction of local structures in globular proteins. J. Mol. Biol. 213, 859–883.
Hendlich M., Lackner P., Weitckus S., Floeckner H., Froschauer R., Gottsbacher K., Casari G., Sippl M.J. 1990. Identification of native protein folds amongst a large number of incorrect models: The calculation of low energy conformations from potentials of mean force. J. Mol. Biol. 216, 167–180.
Godzik A., Kolinski A., Skolnik J. 1992. Topology fingerprint approach to the inverse protein folding problem. J. Mol. Biol. 227, 227–238.
Jones D.T., Thornton J.M. 1996. Potential energy functions for threading. Curr. Opin. Struct. Biol. 6, 210–216.
Lobanov M.Yu., Bogatyreva N.S., Ivankov D.N., Finkelstein A.V. 2009. Prediction of protein structure by analogy: 1. Now database of spatially similar and dissimilar protein domain structures for testing and optimizing prodiction methods. Mol. Biol. 43, 665–676.
Gonnet G.H., Cohen M.A., Benner S.A. 1992. Exhaustive matching of the entire protein sequence database. Science. 256, 1443–1445.
Henikoff S., Henikoff J.G. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA. 89, 10915–10919.
Miyazawa S., Jernigan R.L. 1993. A new substitution matrix for protein sequence searches based on contact frequencies in protein structures. Protein Eng. 6, 267–278.
Miyazawa S., Jernigan R.L. 1996. Residue-residue potentials with a favorable contact pair term and an unfavorable high packing density term, for simulation and threading. J. Mol. Biol. 256, 623–644.
Miyazawa S., Jernigan R.L. 1999. Self-consistent estimation of inter-residue protein contact energies based on an equilibrium mixture approximation of residues. Proteins. 34, 49–68.
Berman H., Henrick K., Nakamura H., Markley J.L. 2007. The worldwide Protein Data Bank (wwPDB): Ensuring a single, uniform archive of PDB data. Nucleic Acid Res. 35, D301–D303; http://www.wwpdb.org.
Kabsch W., Sander C. 1983. Dictionary of protein secondary structure: Pattern recognition of hydrogenbonded and geometrical features. Biopolymers. 22, 2577–2637; http://mobyle.pasteur.fr/cgi-bin/Mobyle-Portal/portal.py?form = dssp.
Jones D. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol Biol. 292, 195–202; http://bioinf.cs.ucl.ac.uk/psipred.
Finkelstein A.V., Badretdinov A.Ya., Gutin A.M. 1995. Why do protein architectures have a Boltzmann-like statistics? Proteins. 23, 142–150.
Murzin A.G., Brenner S.E., Hubbard T., Chothia C. 1995. SCOP: A structural classification of proteins database for the investigation of sequences and structures. J. Mol. Biol. 247, 536–540; http://scop.mrclmb.cam.ac.uk/scop/parse/index.html.
Siew N., Elofsson A., Rychlewski L., Fischer D. 2000. MaxSub: An automated measure for the assessment of protein structure prediction quality. Bioinformatics. 16, 776–785.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.Yu. Lobanov, A.V. Finkel’shtein, 2009, published in Molekulyarnaya Biologiya, 2009, Vol. 43, No. 4, pp. 733–740.
Rights and permissions
About this article
Cite this article
Lobanov, M.Y., Finkel’shtein, A.V. Analogy-based protein structure prediction: II. Testing of substitution matrices and pseudopotentials used to align protein sequences with spatial structures. Mol Biol 43, 677–684 (2009). https://doi.org/10.1134/S0026893309040207
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893309040207