Abstract
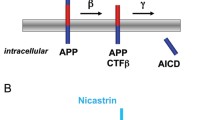
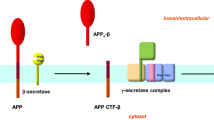
Studies of the molecular mechanisms of Alzheimer’s disease (AD) have led to two major achievements. First, genes with mutations causing AD (PS1 and PS2 presenilin genes and APP) or bearing a risk factor polymorphism (APOE) have been described. Second, a new type of proteases has been identified along with the mechanisms regulating cell differentiation and development via intramembrane proteolysis. These mechanisms are apparently universal for various cell systems and organisms. Presenilin is a catalytic component of the tetraprotein complex (ɛ-/γ-secretase) that cleaves type I transmembrane proteins. Type II transmembrane proteins are cleaved by IMPAS/SPP aspartate proteases, found recently. The processing of transmembrane proteins by intramembrane proteases generates signal peptides, transcription factors, and short hydrophobic proteins (fragments of transmembrane domains), which can play both a physiological role and a key role in pathological events associated with aging (β-amyloid in AD). About 160 PS1 mutations, more than ten PS2 mutations, and 21 APP mutations have been described to date. Preclinical diagnosis has become possible for some early-onset forms of AD. Since early-and late-onset forms of AD are similar in pathogenesis, studies of proteolysis driven by intramembrane aspartate proteases may eventually contribute to the development of drugs directly affecting the processing of transmembrane proteins as a primary mechanism of AD.
Similar content being viewed by others
References
Ferri C.P., Prince M., Brayne C., et al. 2005. Global prevalence of dementia: A Delphi consensus study. Lancet. 366, 2112–2117.
Serpell L.C. 2000. Alzheimer’s amyloid fibrils: Structure and assembly. Biochim. Biophys. Acta. 1502, 16–30.
Glenner G.G., Wong C.W. 1984. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890.
Glenner G.G., Wong C.W. 1984. Alzheimer’s disease and Down’s syndrome: Sharing of a unique cerebrovascular amyloid fibril protein. Biochem. Biophys. Res. Commun. 122, 1131–1135.
Goldgaber D., Lerman M.I., McBride O.W., et al. 1987. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science. 235, 877–880.
Kang J., Lemaire H.G., Unterbeck A., et al. 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 325, 733–736.
Goate A., Chartier-Harlin M.C., Mullan M., et al. 1991. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 349, 704–706.
Hardy J.A., Higgins G.A. 1992. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 256, 184–185.
Hardy J. 2003. The Genetics of Alzheimer’s Disease. In: Alzheimer’s Disease and Related Disorders: Research Advances. Eds. Iqbal K., Winblad B. Bucharest: Ana. Aslan. Intl. Acad. of Aging, 29–47.
St. George-Hyslop P., Haines J., Rogaev E., et al. 1992. Genetic evidence for a novel familial Alzheimer’s disease locus on chromosome 14. Nature Genet. 2, 330–334.
Schellenberg G.D., Bird T.D., Wijsman E.M., et al. 1992. Genetic linkage evidence for a familial Alzheimer’s disease locus on chromosome 14. Science. 258, 668–671.
Mullan M., Houlden H., Windelspecht M., et al. 1992. A locus for familial early-onset Alzheimer’s disease on the long arm of chromosome 14, proximal to the α1-antichymotrypsin gene. Nature Genet. 2, 340–342.
Van Broeckhoven C., Backhovens H., Cruts M., et al. 1992. Mapping of a gene predisposing to early-onset Alzheimer’s disease to chromosome 14q24.3. Nature Genet. 2, 335–339.
Rogaev E.I., Sherrington R., Rogaeva E.A., et al. 1995. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 376, 775–778.
Sherrington R., Rogaev E.I., Liang Y., et al. 1995. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 375, 754–760.
Levy-Lahad E., Wasco W., Poorkaj P., et al. 1995. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 269, 973–977.
The Alzheimer Research Forum http://www.alzforum.org/res/com/mut/default.asp
Alzheimer Disease & Frontotemporal Dementia Mutation Database http://www.molgen.ua.ac.be/ admutations/
Crook R., Verkkoniemi A., Perez-Tur J., et al. 1998. A variant of Alzheimer’s disease with spastic paraparesis and unusual plaques due to deletion of exon 9 of presenilin 1. Nature Med. 4, 452–455.
Pericak-Vance M.A., Bebout J.L., Gaskell P.C. Jr., et al. 1991. Linkage studies in familial Alzheimer disease: Evidence for chromosome 19 linkage. Am. J. Hum. Genet. 48, 1034–1050.
Corder E.H., Saunders A.M., Strittmatter W.J., et al. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 261, 921–923.
Chartier-Harlin M.C., Parfitt M., Legrain S., et al. 1994. Apolipoprotein E, ɛ4 allele as a major risk factor for sporadic early-and late-onset forms of Alzheimer’s disease: Analysis of the 19q13.2 chromosomal region. Human Mol. Genet. 3, 569–574.
Rogaev E.I. Genetic factors and a polygenic model of Alzheimer’s disease. Genetika. 35, 1558–1571.
Riazanskaia N., Lukiw W.J., Grigorenko A., et al. 2002. Regulatory region variability in the human presenilin-2 (PSEN2) gene: Potential contribution to the gene activity and risk for AD. Mol. Psychiatry. 7, 891–898.
Corder E.H., Saunders A.M., Risch N.J., et al. 1994. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genet. 7, 180–184.
Lesne S., Koh M.T., Kotilinek L., et al. 2006. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 440, 352–357.
Naslund J., Haroutunian V., Mohs R., et al. 2000. Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. J. Am. Med. Ass. 283, 1571–1577.
Grundke-Iqbal I., Iqbal K., Tung Y.C., et al. 1986. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 83, 4913–4917.
Baker M., Litvan I., Houlden H., et al. 1999. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Human Mol. Genet. 8, 711–715.
Theuns J., Brouwers N., Engelborghs S., et al. 2006. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am. J. Hum. Genet. 78, 936–946.
Bertram L., Blacker D., Mullin K., et al. 2000. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 290, 2302–2303.
Pericak-Vance M.A., Bass M.P., Yamaoka L.H., et al. 1997. Complete genomic screen in late-onset familial Alzheimer disease: Evidence for a new locus on chromosome 12. J. Am. Med. Assoc. 278, 1237–1241.
Kehoe P., Wavrant-De Vrieze V.F., Crook R., et al. 1999. A full genome scan for late onset Alzheimer’s disease. Human Mol. Genet. 8, 237–245.
Prasher V.P., Farrer M.J., Kessling A.M., et al. 1998. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann. Neurol. 43, 380–383.
Rovelet-Lecrux A., Hannequin D., Raux G., et al. 2006. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nature Genet. 38, 24–26.
Moliaka Y.K., Grigorenko A., Madera D., Rogaev E.I. 2004. Impas 1 possesses endoproteolytic activity against multipass membrane protein substrate cleaving the presenilin 1 holoprotein. FEBS Lett. 557, 185–192.
Thinakaran G., Borchelt D.R., Lee M.K., et al. 1996. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 17, 181–190.
Moon R.T., Kohn A.D., De Ferrari G.V., Kaykas A. 2004. WNT and β-catenin signalling: Diseases and therapies. Nature Rev. Genet. 5, 691–701.
Mattson M.P., Chan S.L. 2003. Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium. 34, 385–397.
Scheuner D., Eckman C., Jensen M., et al. 1996. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nature Med. 2, 864–870.
Kimberly W.T., Zheng J.B., Guenette S.Y., Selkoe D.J. 2001. The intracellular domain of the β-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J. Biol. Chem. 276, 40,288–40,292.
Cao X., Sudhof T.C. 2004. Dissection of amyloid-β precursor protein-dependent transcriptional transactivation. J. Biol. Chem. 279, 24,601–24,611.
Borchelt D.R., Thinakaran G., Eckman C.B., et al. 1996. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Aβ1-42/1-40 ratio in vitro and in vivo. Neuron. 17, 1005–1013.
De Strooper B., Saftig P., Craessaerts K., et al. 1998. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 391, 387–390.
Zhang Z., Nadeau P., Song W., et al. 2000. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nature Cell Biol. 2, 463–465.
Wolfe M.S., Xia W., Ostaszewski B.L., et al. 1999. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 398, 513–517.
Xia X., Wang P., Sun X., et al. 2002. The aspartate-257 of presenilin 1 is indispensable for mouse development and production of β-amyloid peptides through β-catenin-independent mechanisms. Proc. Natl. Acad. Sci. USA. 99, 8760–8765.
Steiner H., Duff K., Capell A., et al. 1999. A loss of function mutation of presenilin-2 interferes with amyloid β-peptide production and Notch signaling. J. Biol. Chem. 274, 28,669–28,673.
Tomita T., Watabiki T., Takikawa R., et al. 2001. The first proline of PALP motif at the C terminus of presenilins is obligatory for stabilization, complex formation, and γ-secretase activities of presenilins. J. Biol. Chem. 276, 33273–33281.
Jankowsky J.L., Fadale D.J., Anderson J., et al. 2004. Mutant presenilins specifically elevate the levels of the 42-residue β-amyloid peptide in vivo: Evidence for augmentation of a 42-specific γ-secretase. Human Mol. Genet. 13, 159–170.
Li X., Greenwald I. 1997. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc. Natl. Acad. Sci. USA. 94, 12204–12209.
Levitan D., Greenwald I. 1998. Effects of SEL-12 presenilin on LIN-12 localization and function in Caenorhabditis elegans. Development. 125, 3599–3606.
Shen J., Bronson R.T., Chen D.F., et al. 1997. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 89, 629–639.
Herreman A., Hartmann D., Annaert W., et al. 1999. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc. Natl. Acad. Sci. USA. 96, 11,872–11,877.
Swiatek P.J., Lindsell C.E., del Amo F.F., et al. 1994. Notch1 is essential for postimplantation development in mice. Genes Dev. 8, 707–719.
De Strooper B., Annaert W., Cupers P., et al. 1999. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 398, 518–522.
Takasugi N., Tomita T., Hayashi I., et al. 2003. The role of presenilin cofactors in the γ-secretase complex. Nature. 422, 438–441.
Iso T., Kedes L., Hamamori Y. 2003. HES and HERP families: Multiple effectors of the Notch signaling pathway. J. Cell Physiol. 194, 237–255.
Vetrivel K.S., Zhang Y.W., Xu H., Thinakaran G. 2006. Pathological and physiological functions of presenilins. Mol. Neurodegener. 1, 4.
Yu G., Chen F., Levesque G., et al. 1998. The presenilin 1 protein is a component of a high molecular weight intracellular complex that contains γ-catenin. J. Biol. Chem. 273, 16,470–16,475.
Thinakaran G., Harris C.L., Ratovitski T., et al. 1997. Evidence that levels of presenilins (PS1 and PS2) are coordinately regulated by competition for limiting cellular factors. J. Biol. Chem. 272, 28,415–28,422.
Li Y.M., Lai M.T., Xu M., et al. 2000. Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc. Natl. Acad. Sci. USA. 97, 6138–6143.
Li Y.M., Xu M., Lai M.T., et al. 2000. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 405, 689–694.
Yu G., Nishimura M., Arawaka S., et al. 2000. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 407, 48–54.
Goutte C., Hepler W., Mickey K.M., Priess J.R. 2000. aph-2 encodes a novel extracellular protein required for GLP-1-mediated signaling. Development. 127, 2481–2492.
Shah S., Lee S.F., Tabuchi K., et al. 2005. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 122, 435–447.
Edbauer D., Winkler E., Haass C., Steiner H. 2002. Presenilin and nicastrin regulate each other and determine amyloid β-peptide production via complex formation. Proc. Natl. Acad. Sci. USA. 99, 8666–8671.
Kimberly W.T., laVoie M.J., Ostaszewski B.L., et al. 2002. Complex N-linked glycosylated nicastrin associates with active γ-secretase and undergoes tight cellular regulation. J. Biol. Chem. 277, 35,113–35,117.
Goutte C., Tsunozaki M., Hale V.A., Priess J.R. 2002. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc. Natl. Acad. Sci. USA. 99, 775–779.
Francis R., McGrath G., Zhang J., et al. 2002. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev.Cell. 3, 85–97.
Lee S.F., Shah S., Li H., et al., et al. 2002. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-β precursor protein and Notch. J. Biol. Chem. 277, 45013–45019.
Luo W.J., Wang H., Li H., et al. 2003. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J. Biol. Chem. 278, 7850–7854.
Gu Y., Chen F., Sanjo N., et al. 2003. APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin-nicastrin complexes. J. Biol. Chem. 278, 7374–7380.
Edbauer D., Winkler E., Regula J.T., et al. 2003. Reconstitution of γ-secretase activity. Nature Cell Biol. 5, 486–488.
Fraering P.C., Ye W., Strub J.M., et al. 2004. Purification and characterization of the human γ-secretase complex. Biochemistry. 43, 9774–9789.
Rogaev E.I., Grigorenko A., Ryazanskaya N., et al. Evolution and modulation of Alzheimer’s disease associated genes and families of related proteins. 6th Annual International Human Genome Meeting. 2001. Edinburgh, UK. Program and Abstract Book. p. 28. Abstract 101.
Grigorenko A.P., Molyaka Yu.K., Korovaitseva G.I., Rogaev E.I. 2002. Identification of a novel class of polytopic proteins with domains associated with probable proteinase activity. Biokhimiya. 67, 995–1005.
Weihofen A., Binns K., Lemberg M.K., et al. 2002. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science. 296, 2215–2218.
Grigorenko A.P., Moliaka Y.K., Soto M.C., et al. 2004. The Caenorhabditis elegans IMPAS gene, imp-2, is essential for development and is functionally distinct from related presenilins. Proc. Natl. Acad. Sci. USA. 101, 14955–14960.
Nicoll J.A., Wilkinson D., Holmes C., et al. 2003. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: A case report. Nature Med. 9, 448–452.
Siemers E.R., Quinn J.F., Kaye J., et al. 2006. Effects of a γ-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 66, 602–604.
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.P. Grigorenko, E.I. Rogaev, 2007, published in Molekulyarnaya Biologiya, 2007, Vol. 41, No. 2, pp. 331–345.
Rights and permissions
About this article
Cite this article
Grigorenko, A.P., Rogaev, E.I. Molecular basis of Alzheimer’s disease. Mol Biol 41, 294–307 (2007). https://doi.org/10.1134/S0026893307020100
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S0026893307020100