Abstract
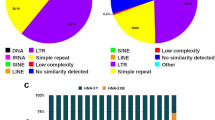
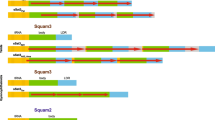
Copies of two repetitive elements of the common tree shrew (Tupaia glis) genome were cloned and sequenced. The first element, Tu III, is a ∼260 bp long short interspersed element (SINE) with the 5′ end derived from glycine RNA. Tu III carries long polypurine-and polypyrimidine-rich tracts, which may contribute to the specific secondary structure of Tu III RNA. This SINE was also found in the genome of the smooth-tailed tree shrew of another genus (Dendrogale). Tu III appears to be confined to the order Scandentia since it was not found in the DNA of other tested mammals. The second element, Tu-SAT1, is a tandem repeat with a monomer length of 365 bp. Some properties of its nucleotide sequence suggest that Tu-SAT1 is a centromeric satellite.
Similar content being viewed by others
References
Beridze T.G. 1982. Satellitnaya DNK (Sattelite DNA). Moscow: Nauka.
Willard H.F. 1990. Centromeres of mammalian chromosomes. Trends Genet. 6, 410–416.
Elder J.F., Jr., Turner B.J. 1995. Concerted evolution of repetitive DNA sequences in eukaryotes. Quant. Rev. Biol. 70, 297–320.
Liao D. 1999. Concerted evolution: Molecular mechanism and biological implications. Am. J. Hum. Genet. 64, 24–30.
Henikoff S., Ahmad K., Malik H.S. 2001. The centromere paradox: Stable inheritance with rapidly evolving DNA. Science. 293, 1098–1102.
Ugarkovic D., Plohl M. 2002. Variation in satellite DNA profiles: Causes and effects. EMBO J. 21, 5955–5959.
Lamb J.C., Birchler J.A. 2003. The role of DNA sequence in centromere formation. Genome Biol. 4, 214.
Enukashvili N.I., Kuznetsova I.S., Podgornaya O.I. 2003. The mammalian centromere organization. Tsitologiya. 45, 255–270.
Smit A.F. 1999. Interspersed repeats and other mementos of transposable elements in mammalian genomes. Current Opin. Genet. Dev. 9, 657–663.
Deininger P.L., Batzer M.A. 2002. Mammalian retroelements. Genome Res. 12, 1455–1465.
Kramerov D.A., Vassetzky N.S. 2005. Short retroposons (SINEs) in eukaryotic genomes. Int. Rev. Cytol. 247, 165–221.
Orgel L.E., Crick F.H. 1980. Selfish DNA: The ultimate parasite. Nature. 284, 604–607.
Makalowski W. 2000. Genomic scrap yard: How genomes utilize all that junk. Gene. 259, 61–67.
Allen T.A., von Kaenel S., Goodrich J.A., Kugel J.F. 2004. The SINE-encoded mouse B2 RNA represses mRNA transcription in response to heat shock. Nature Struct. Mol. Biol. 11, 816–821.
Serdobova I.M., Kramerov D.A. 1994. Use of short retroposons as phylogenetic markers. Dokl. Akad. Nauk. 335, 664–667.
Shimamura M., Yasue H., Ohshima K., Abe H., Kato H., Kishiro T., Goto M., Munechika I., Okada N. 1997. Molecular evidence from retroposons that whales form a clade within even-toed ungulates. Nature. 388, 666–670.
Shedlock A.M., Okada N. 2000. SINE insertions: Powerful tools for molecular systematics. Bioessays. 22, 148–160.
Stoneking M., Fontius J.J., Clifford S.L., Soodyall H., Arcot S.S., Saha N., Jenkins T., Tahir M.A., Deininger P.L., Batzer M.A. 1997. Alu insertion polymorphisms and human evolution: evidence for a larger population size in Africa. Genome Res. 7, 1061–1071.
Kramerov D., Vassetzky N., Serdobova I. 1999. The evolutionary position of dormice (Gliridae) in Rodentia determined by a novel short retroposon. Mol. Biol. Evol. 16, 715–717.
Nikaido M., Nishihara H., Hukumoto Y., Okada N. 2003. Ancient SINEs from African endemic mammals. Mol. Biol. Evol. 20, 522–527.
Vassetzky N.S., Ten O.A., Kramerov D.A. 2003. B1 and related SINEs in mammalian genomes. Gene. 319, 149–160.
Carrol R.L. 1988. Vertebrate Paleontology and Evolution. N.Y.: Freeman and Company.
Pavlinov I.Ya. 2003. Sistematika sovremennykh mlekopitayushchikh (Taxonomy of Recent Mammals), Moscow: Mosk. Gos. Univ.
Borodulina O.R., Kramerov D.A. 2001. Short interspersed elements (SINEs) from insectivores. Two classes of mammalian SINEs distinguished by A-rich tail structure. Mammal. Genome. 12, 779–786.
Redston M.S., Kern S.E. 1994. Klenow co-sequencing: A method for eliminating “stops”. Biotechniques. 17, 286–288.
Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415.
Sarai A., Mazur J., Nussinov R., Jernigan R.L. 1989. Sequence dependence of DNA conformational flexibility. Biochemistry. 28, 7842–7849.
Gromiha M. 2000. Structure based sequence dependent stiffness scale for trinucleotides: A direct method. J. Biol. Phys. 26, 43–50.
Nishihara H., Terai Y., Okada N. 2002. Characterization of novel Alu-and tRNA-related SINEs from the tree shrew and evolutionary implications of their origins. Mol. Biol. Evol. 19, 1964–1972.
Debrauwere H., Gendrel C.G., Lechat S., Dutreix M. 1997. Differences and similarities between various tandem repeat sequences: Minisatellites and microsatellites. Biochimie. 79, 577–586.
Masumoto H., Nakano M., Ohzeki J. 2004. The role of CENP-B and alpha-satellite DNA: De novo assembly and epigenetic maintenance of human centromeres. Chromosome Res. 12, 543–556.
Schattner P., Brooks A.N., Lowe T.M. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33, W686–W689.
Author information
Authors and Affiliations
Additional information
Original Russian Text © O.A. Ten, O.R. Borodulina, N.S. Vassetzky, N.In. Oparina, D.A. Kramerov, 2006, published in Molekulyarnaya Biologiya, 2006, Vol. 40, No. 1, pp. 74–83.
Rights and permissions
About this article
Cite this article
Ten, O.A., Borodulina, O.R., Vassetzky, N.S. et al. Repetitive sequences of the tree shrew genome (Mammalia, Scandentia). Mol Biol 40, 63–71 (2006). https://doi.org/10.1134/S0026893306010109
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0026893306010109