Abstract—
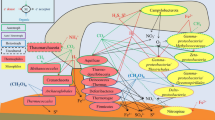
Seasonal cyanobacterial blooms have a negative impact on freshwater ecosystems. The role of cyanobacteria in methane production and their relationship with methanogenic archaea are not yet well understood. The goal of the present work was to identify the features of methanogenesis in the water column and sediments of a profundal part of the freshwater Lake Senezh (Moscow oblast) during a period of cyanobacterial over-bloom. Analytical, radiotracer, microscopic, molecular biological, and incubation techniques were used. Alkalization and oxygen oversaturation of the 0‒2-m water layer were caused by intensive photosynthesis. The near-bottom water (4 m) was pH-neutral and hypoxic; the sediments were reduced. Methane was detected throughout the water column; its concentration in the surface water was an order of magnitude lower than in the near-bottom water and 4 orders of magnitude lower than in the sediments. Cyanobacteria of the species Microcystis aeruginosa predominated in the photic zone (up to 30% of the total number of the 16S rRNA gene fragments). The sequences of cyanobacteria and freshwater members of the SAR11 clade, which can potentially be involved in aerobic methanogenesis via decomposition of methylphosphonates (MPn), were also detected. The sequences of hydrogenotrophic methanogens of the genus Methanoregula, which are potentially capable of methanogenesis in cooperation with cyanobacteria, were revealed in oxygen-supersaturated water. Hydrogenotrophic and acetoclastic pathways of methanogenesis predominated in reduced sediments. Sequences of methanogens of the orders Methanomicrobiales, Methanobacteriales, Methanosarciniales, and Methanomassiliicoccales were detected there. Cyanobacterial bloom promoted methanogenesis both in the photic zone of Lake Senezh (due to MPn decomposition and anaerobic methanogenesis in association with cyanobacterial aggregates) and in the near-bottom water and sediments (due to oxygen depletion and excessive release of substrates caused by sedimentation and degradation of cyanobacterial mortmass).




Similar content being viewed by others
REFERENCES
Asada, Y. and Kawamura, S., Hydrogen evolution by Microcystis aeruginosa in darkness, Agric. Biol. Chem., 1984, vol. 48, pp. 2595−2596.
Asada, Y., Miyake, M., Koike, Y., Aoyama, K., Uemura, I., and Miyake, J., Hydrogenase-mediated hydrogen metabolism in a non-nitrogen-fixing cyanobacterium, Microcystis aeruginosa, in BioHydrogen, Zaborsky, O.R., Benemann, J.R., Matsunaga, T., Miyake, J., and San Pietro, A., Eds., Boston: Springer, 1998, pp. 173−179. https://doi.org/10.1007/978-0-585-35132-2_23
Berg, A., Lindblad, P., and Svensson, B.H., Cyanobacteria as a source of hydrogen for methane formation, World J. Microbiol. Biotechnol., 2014, vol. 30, pp. 539−545.
Bižic, M., Grossart, H.-P., and Ionescu, D., Methane paradox, in eLS, Chichester: John Wiley & Sons, 2020, pp. 1‒11. https://doi.org/10.1002/9780470015902.a0028892
Borrel, G., Jézéquel, D., Biderre-Petit, C., Morel-Desrosiers, N., Morel, J.P., Peyret, P., Fonty, G., and Lehours, A.C., Production and consumption of methane in freshwater lake ecosystems, Res. Microbiol., 2011, vol. 162, pp. 832−847.
Carini, P., White, A.E., Campbell, E.O., and Giovannoni, S.J., Methane production by phosphate-starved SAR11 chemoheterotrophic marine bacteria, Nat. Commun., 2014, vol. 5, p. 4346. https://doi.org/10.1038/ncomms5346
Diaz, R.J. and Rosenberg, R., Spreading dead zones and consequences for marine ecosystems, Science, 2008, vol. 321, pp. 926−929.
Edgar, R.C., Search and clustering orders of magnitude faster than BLAST, Bioinformatics, 2010, vol. 26, pp. 2460−2461.
Frey, B., Rime, T., Phillips, M., Stierli, B., Hajdas, I., Widmer, F., and Hartmann, M., Microbial diversity in European alpine permafrost and active layers, FEMS Microbiol. Ecol., 2016, vol. 92, p. fiw018. https://doi.org/10.1093/femsec/fiw018
Henson, M.W., Lanclos, V.C., Faircloth, B.C., and Thrash, J.C., Cultivation and genomics of the first freshwater SAR11 (LD12) isolate, ISME J., 2018, vol. 12, pp. 1846−1860.
Huisman, J., Codd, G.A., Paerl, H.W., Ibelings, B.W., Verspagen, J.M.H., and Visser, P.M., Cyanobacterial blooms, Nat. Rev. Microbiol., 2018, vol. 16, pp. 471–483.
Kallistova, A., Kadnikov, V., Rusanov, I., Kokryatskaya, N., Beletsky, A., Mardanov, A., Savvichev, A., Ravin, N., and Pimenov, N., Microbial communities involved in aerobic and anaerobic methane cycling in a meromictic ferruginous subarctic lake, Aquatic Microb. Ecol., 2018, vol. 82, pp. 1−18.
Kallistova, A.Yu., Kadnikov, V.V., Savvichev, A.S., Rusanov, I.I., Dvornikov, Yu.A., Leibman, M.O., Kho-mutov, A.V., Ravin, N.V., and Pimenov, N.V., Comparative study of methanogenic pathways in the sediments of thermokarst and polygenetic Yamal lakes, Microbiology (Moscow), 2021, vol. 90, pp. 261–267.
Kallistova, A.Yu., Koval, D.D., Kadnikov, V.V., Toshchakov, S.V., Yusupov, S.K., Izotova, A.O., Vinogrado-va, E.N., Zekker, I., and Pimenov, N.V., Methane cycle in a littoral site of a temperate freshwater lake, Microbiology (Moscow), 2023, vol. 92, pp. 153–170.
Li, C., Hambright, K.D., Bowen, H.G., Trammell, M.A., Grossart, H.P., Burford, M.A., Hamilton, D.P., Jiang, H., Latour, D., Meyer, E.I., Padisák, J., Zamor, R.M., and Krumholz, L.R., Global co-occurrence of methanogenic archaea and methanotrophic bacteria in Microcystis aggregates, Environ. Microbiol., 2021, vol. 23, pp. 6503−6519.
Lyautey, E., Billard, E., Tissot, N., Jacquet, S., and Domaizon, I., Seasonal dynamics of abundance, structure, and diversity of methanogens and methanotrophs in lake sediments, Microb. Ecol., 2021, vol. 82, pp. 559−571.
Lyu, Z. and Lu, Y., Metabolic shift at the class level sheds light on adaptation of methanogens to oxidative environments, ISME J., 2018, vol. 12, pp. 411−423.
Magoč, T. and Salzberg, S.L., FLASH: fast length adjustment of short reads to improve genome assemblies, Bioinformatics, 2011, vol. 27, pp. 2957−2963.
McAuliffe, C.C., GC determination of solutes by multiple phase equilibration, Chem. Technol., 1971, vol. 1, pp. 46−51.
Namsaraev, Z.B., Application of extinction coefficients for quantification of chlorophylls and bacteriochlorophylls, Microbiology (Moscow), 2009, vol. 78, pp. 794–797.
Peura, S., Sinclair, L., Bertilsson, S., and Eiler, A., Metagenomic insights into strategies of aerobic and anaerobic carbon and nitrogen transformation in boreal lakes, Sci. Rep., 2015, vol. 5, p. 12102. https://doi.org/10.1038/srep12102
Rashid, N., Song, W., Park, J., Jin, H.-F., and Lee, K., Characteristics of hydrogen production by immobilized cyanobacterium Microcystis aeruginosa through cycles of photosynthesis and anaerobic incubation, Ind. Eng. Chem., 2009, vol. 15, pp. 498−503.
Reinl, K.L., Harris, T.D., North, R.L., Almela, P., Berger, S.A., Bizic, M., Burnet, S.H., Grossart, H.-P., Ibelings, B.W., Jakobsson, E., Knoll, L.B., Lafran-cois, B.M., McElarney, Y., Morales-Williams, A.M., Obertegger, U., et al., Blooms also like it cold, Limnol. Oceanogr. Lett., 2023. https://doi.org/10.1002/lol2.10316
Rissanen, A.J., Karvinen, A., Nykänen, H., Peura, S., Tiirola, M., Mäki, A., and Kankaala, P., Effects of alternative electron acceptors on the activity and community structure of methane-producing and consuming microbes in the sediments of two shallow boreal lakes, FEMS Microbiol. Ecol., 2017, vol. 93, p. fix078. https://doi.org/10.1093/femsec/fix078
Rognes, T., Flouri, T., Nichols, B., Quince, C., and Mahé, F., VSEARCH: a versatile open source tool for metagenomics, PeerJ, 2016, vol. 4, p. e2584. https://doi.org/10.7717/peerj.2584
Samylina, O.S., Merkel, A.Y., and Pimenov, N.V., Diurnal methane dynamics in the cyanobacterial community of soda Lake Bitter 1 (Kulunda Steppe, Altai Krai), Microbiology (Moscow), 2023, vol. 92, pp. 293–299.
Smucker, N.J., Beaulieu, J.J., Nietch, C.T., and Young, J.L., Increasingly severe cyanobacterial blooms and deep water hypoxia coincide with warming water temperatures in reservoirs, Glob. Change Biol., 2021, vol. 27, pp. 2507−2519.
Sorokin, Y.I. and Kadota, H., Techniques for the assessment of microbial production and decomposition in fresh waters, in IBP Handbook 23, Oxford and London: Blackwell Scientific Publ., 1972.
Stal, L.J., Nitrogen fixation in cyanobacteria, in eLS, Chichester: John Wiley & Sons, 2015, pp. 1‒9. https://doi.org/10.1002/9780470015902.a0021159.pub2
Tsementzi, D., Rodriguez-R, L.M., Ruiz-Perez, C.A., Meziti, A., Hatt, J.K., and Konstantinidis, K.T., Ecogenomic characterization of widespread, closely-related SAR11 clades of the freshwater genus “Candidatus Fonsibacter” and proposal of Ca. Fonsibacter lacus sp. nov., Syst. Appl. Microbiol., 2019, vol. 42, pp. 495−505.
Wetzel, R.G., Limnology: Lake and River Ecosystems, Academic Press, 2001, 3rd ed. https://doi.org/10.1016/C2009-0-02112-6.
Wilhelm, S.W., Bullerjahn, G.S., and McKay, R.M.L., The complicated and confusing ecology of Microcystis blooms, mBio, 2020, vol. 11, p. e00529-20. https://doi.org/10.1128/mBio.00529-20
Xu, H., Liu, Y., Tang, Z., Li, H., Li, G., and He, Q., Methane production in harmful algal blooms collapsed water: The contribution of non-toxic Microcystis aeruginosa outweighs that of the toxic variety, J. Cleaner Product., 2020, vol. 276, p. 124280. https://doi.org/10.1016/j.jclepro.2020.124280
Zhao, L., Lin, L.Z., Chen, M.Y., Teng, W.K., Zheng, L.L., Peng, L., Lv, J., Brand, J.J., Hu, C.X., Han, B.P., Song, L.R., and Shu, W.S., The widespread capability of methylphosphonate utilization in filamentous cyanobacteria and its ecological significance, Water Res., 2022, vol. 217, p. 118385. https://doi.org/10.1016/j.watres.2022.118385
Zhu, Y., Chen, X., Yang, Y., and Xie, S., Impacts of cyanobacterial biomass and nitrate nitrogen on methanogens in eutrophic lakes, Sci. Total. Environ., 2022, vol. 848, p. 157570. https://doi.org/10.1016/j.scitotenv.2022.157570
Funding
This study was partially funded by the Russian Science Foundation, grant no. 22-14-00038 and by the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interests.
Statement on the welfare of animals. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by D. Timchenko
Supplementary Information
Rights and permissions
About this article
Cite this article
Kallistova, A.Y., Kosyakova, A.I., Rusanov, I.I. et al. Methane Production in a Temperate Freshwater Lake during an Intense Cyanobacterial Bloom. Microbiology 92, 638–649 (2023). https://doi.org/10.1134/S0026261723601586
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261723601586