Abstract—
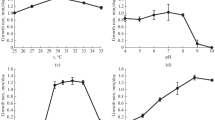
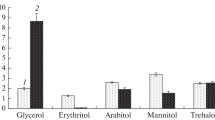
Investigation of the growth rate of Sistotrema brinkmannii at different pH values, temperature, and NaCl concentration showed that this fungus was a mesophile, preferred a salt-free medium, and was an obligate acidophile, since it had a pronounced growth optimum at pH 3.0–4.0 and did not grow at pH 7.0. To reveal the protective mechanisms allowing this fungus to develop under acidic conditions, the composition of its osmolytes and lipids was studied. This is the first report on occurrence of a large amount of trehalose (4.0‒6.6% of dry weight) in the mycelium of the fungus during its growth under optimal conditions, confirming the use of osmolytes by acidophiles for adaptation. At the same time, at the borders of the growth range (pH 2.6 and 6.0), the amount of trehalose in the mycelium of the fungus decreased by 2.5 times, which was in agreement with a narrow growth optimum of the fungus in its natural environments (pH 3.0–4.0). The composition of membrane lipids of the fungus was characterized by a high proportion of sphingolipids (up to 60% of the total), which decreased twofold during growth under optimal conditions. The main membrane lipids, apart from sphingolipids, were phosphatidic acids, phosphatidylethanolamines, and sterols; the proportion of these lipids increased with time. The composition of membrane lipids of the fungus at pH 2.6 did not differ much from the optimal conditions, while in the near-neutral region there was a twofold increase in the proportion of sphingolipids, indicating their adaptive value. The simultaneous decrease in the proportion of sphingolipids and the increase in the level of trehalose in the growth dynamics suggest association of these compounds in the protection of cell membranes.




Similar content being viewed by others
REFERENCES
Agnew, M.P., Craigie, C.R., Weralupitiya, G., Reis, M.M., Johnson, P.L., and Reis, M.G., Comprehensive evaluation of parameters affecting one-step method for quantitative analysis of fatty acids in meat, Metabolites, 2019, vol. 9, art. 189, pp. 1‒15. https://doi.org/10.3390/metabo9090189
Aguilera, A. and González-Toril, E., Eukaryotic life in extreme environments: acidophilic fungi, in Fungi in Extreme Environments: Ecological Role and Biotechnological Significance, Cham: Springer Int., 2019, pp. 21–38. https://doi.org/10.1007/978-3-030-19030-9_2
Amaral-Zettler L.A. Eukaryotic diversity at pH extremes, Front. Microbiol. 2012, vol. 3, art. 00441, pp. 1–17. https://doi.org/10.3389/fmicb.2012.00441
Baker-Austin, C. and Dopson, M., Life in acid: pH homeostasis in acidophiles, Trends Microbiol., 2007, vol. 15, pp. 165–171. https://doi.org/10.1016/j.tim.2007.02.005
Baker, B.J., Tyson, G.W., Goosherst, L., and Banfield, J.F., Insights into the diversity of eukaryotes in acid mine drainage biofilm communities, Appl. Environ. Microbiol., 2009, vol. 75, pp. 2192–2199. https://doi.org/10.1128/AEM.02500-08
Bondarenko, S.A., Ianutsevich, E.A., Danilova, O.A., Grum-Grzhimaylo, A.A., Kotlova, E.R., Kamzolkina, O.V., Bilanenko, E.N., and Tereshina, V.M., Membrane lipids and soluble sugars dynamics of the alkaliphilic fungus Sodiomyces tronii in response to ambient pH, Extremophiles, 2017, vol. 21, pp. 743–754. https://doi.org/10.1007/s00792-017-0940-4
Brobst K.M., Gas–liquid chromatography of trimethylsilyl derivatives: analysis of corn syrup, in General Carbohydrate Methods, Whistler, R.L. and BeMiller, J.N., Eds., New York: Academic, 1972, pp. 3–8.
Brown, A.D. and Simpson, J.R., Water relations of sugar-tolerant yeasts: the role of intracellular polyols, J. Gen. Microbiol., 1972, vol. 72, pp. 589–591. https://doi.org/10.1099/00221287-72-3-589
Coker, J.A., Recent advances in understanding extremophiles, F1000Research, 2019, vol. 8, p. 1917. https://doi.org/10.12688/f1000research.20765.1
Coleine, C., Stajich, J.E., and Selbmann, L., Fungi are key players in extreme ecosystems, Trends Ecol. Evol., 2022, vol. 37, pp. 517–528. https://doi.org/10.1016/j.tree.2022.02.002
Costenoble, R., Valadi, H., Gustafsson, L., Niklasson, C., and Johan Franzen, C., Microaerobic glycerol formation in Saccharomyces cerevisiae, Yeast, 2000, vol. 16, pp. 1483–1495. https://doi.org/10.1002/1097-0061(200012)16:16<1483::AID-YEA642>3.0.CO;2-K
Danilova, O.A., Ianutsevich, E.A., Bondarenko, S.A., Georgieva, M.L., Vikchizhanina, D.A., Groza, N.V., Bilanenko, E.N., and Tereshina, V.M., Osmolytes and membrane lipids in the adaptation of micromycete Emericellopsis alkalina to ambient pH and sodium chloride, Fungal Biol., 2020, vol. 124, pp. 884–891. https://doi.org/10.1016/j.funbio.2020.07.004
Elbein, A.D., Pan, Y.T., Pastuszak, I., and Carroll, D., New insights on trehalose: a multifunctional molecule, Glycobiology, 2003, vol. 13, no. 4, pp. 17R‒27R. https://doi.org/10.1093/glycob/cwg047
Gonçalves, V.N., Vaz, A.B.M., Rosa, C.A., and Rosa, L.H., Diversity and distribution of fungal communities in lakes of Antarctica, FEMS Microbiol. Ecol., 2012, vol. 82, pp. 459–471. https://doi.org/10.1111/j.1574-6941.2012.01424.x
Gross, S. and Robbins, E.I., Acidophilic and acid-tolerant fungi and yeasts, Hydrobiologia, 2000, vol. 433, pp. 91–109. https://doi.org/10.1023/A:1004014603333
Grum-Grzhimaylo, O.A., Debets, A.J.M., and Bilanenko, E.N., The diversity of microfungi in peatlands originated from the White Sea, Mycologia, 2016, vol. 108, pp. 233‒254. https://doi.org/10.3852/14-346
Gunde-Cimerman, N., Plemenitaš, A., and Oren, A., Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations, FEMS Microbiol. Rev., 2018, vol. 42, pp. 353–375. https://doi.org/10.1093/femsre/fuy009
Hallsworth, J.E., Mancinelli, R.L., Conley, C.A., Dallas, T.D., Rinaldi, T., Davila, A.F., Benison, K.C., Rapoport, A., Cavalazzi, B., Selbmann, L., Changela, H., Westall, F., Yakimov, M.M., Amils, R., and Madigan, M.T., Astrobiology of life on Earth, Environ. Microbiol., 2021, vol. 23, pp. 3335–3344. https://doi.org/10.1111/1462-2920.15499
Ianutsevich, E.A., Danilova, O.A., Groza, N.V., Kotlova, E.R., and Tereshina, V.M., Heat shock response of thermophilic fungi: membrane lipids and soluble carbohydrates under elevated temperatures, Microbiology (SGM), 2016, vol. 162, pp. 989–999. https://doi.org/10.1099/mic.0.000279
Ianutsevich, E.A., Danilova, O.A., Kurilov, D.V., Zavarzin, I.V., and Tereshina, V.M. Osmolytes and membrane lipids in adaptive response of thermophilic fungus Rhizomucor miehei to cold, osmotic and oxidative shocks, Extremophiles, 2020, vol. 24, pp. 391–401. https://doi.org/10.1007/s00792-020-01163-3
Inouye, M. and Phadtare, S., Cold-shock response and adaptation to near-freezing temperature in cold-adapted yeasts, in Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance, Buzzini, P. and Margesin, R., Eds., Berlin: Springer Berlin Heidelberg, 2014, pp. 243–257. https://doi.org/10.1007/978-3-642-39681-6
Iturriaga, G., Suárez, R., and Nova-Franco, B., Trehalose metabolism: from osmoprotection to signaling, Int. J. Mol. Sci., 2009, vol. 10, pp. 3793–3810. https://doi.org/10.3390/ijms10093793
Jennings, D.H., Polyol metabolism in fungi, in Advances in Microbial Physiology, Rose, A.H. and Tempest, D.W., Eds., London: Academic, 1985, pp. 149–193. https://doi.org/10.1016/S0065-2911(08)60292-1
Kane, P.M., Proton transport and pH control in fungi, in Yeast Membrane Transport Advances in Experimental Medicine and Biology, Ramos, J., Sychrová, H., and Kschischo, M., Eds., Cham: Springer, 2016, pp. 33–68. https://doi.org/10.1016/S0065-2911(08)60292-1
Kates, M., Techniques of lipidology: isolation, analysis and identification of lipids, in Laboratory Techniques in Biochemistry and Molecular Biology, Work, T.S. and Work, E., Eds., Amsterdam: North-Holland, 1972, pp. 267–610. https://doi.org/10.1016/S0075-7535(08)70544-8
Koide, R.T., Shumway, D.L., and Stevens, C.M., Soluble carbohydrates of red pine (Pinus resinosa) mycorrhizas and mycorrhizal fungi, Mycol. Res., 2000, vol. 104, pp. 834–840. https://doi.org/10.1017/S0953756299002166
Kozlova, M.V., Ianutsevich, E.A., Danilova, O.A., Kamzolkina, O.V., and Tereshina, V.M., Lipids and soluble carbohydrates in the mycelium and ascomata of alkaliphilic fungus Sodiomyces alkalinus, Extremophiles, 2019, vol. 23, pp. 487–494. https://doi.org/10.1007/s00792-019-01100-z
Mattoon, E.R., Casadevall, A., and Cordero, R.J., Beat the heat: correlates, compounds, and mechanisms involved in fungal thermotolerance, Fungal Biol. Rev., 2021, vol. 36, pp. 60–75. https://doi.org/10.1016/j.fbr.2021.03.002
McMahon, H.T. and Gallop, J.L., Membrane curvature and mechanisms of dynamic cell membrane remodelling, Nature, 2005, vol. 438, pp. 590–596. https://doi.org/10.1038/nature04396
Merino, N., Aronson, H.S., Bojanova, D.P., Feyhl-Buska, J., Wong, M.L., Zhang, S., and Giovannelli, D., Living at the extremes: extremophiles and the limits of life in a planetary context, Front. Microbiol., 2019, vol. 10, art. 780. https://doi.org/10.3389/fmicb.2019.00780
Minnikin, D.E., O’Donnell, A.G., Goodfellow, M., Alderson, G., Athalye, M., Schaal, A., and Parlett, J.H., An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids, J. Microbiol. Methods, 1984, vol. 2, pp. 233–241. https://doi.org/10.1016/0167-7012(84)90018-6
Nazareth, S. and Gonsalves, V., Aspergillus penicillioides— a true halophile existing in hypersaline and polyhaline econiches, Ann. Microbiol., 2014, vol. 64, pp. 397–402. https://doi.org/10.1007/s13213-013-0646-5
Nichols, B.W., Separation of the lipids of photosynthetic tissues: improvements in analysis by thin-layer chromatography, Biochim. Biophys. Acta—Spec. Sect. Lipids Relat. Subj., 1963, vol. 70, pp. 417–422. https://doi.org/10.1016/0926-6542(63)90060-X
Péter, M., Gudmann, P., Kóta, Z., Török, Z., Vígh, L., Glatz, A., and Balogh, G., Lipids and trehalose actively cooperate in heat stress management of Schizosaccharomyces pombe, Int. J. Mol. Sci., 2021, vol. 22, art. 13272. https://doi.org/10.3390/ijms222413272
Rothschild, L.J. and Mancinelli, R.L., Life in extreme environments, Nature, 2001, vol. 409, pp. 1092–1101. https://doi.org/10.1038/35059215
Rousk, J., Brookes, P.C., and Bååth, E., Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization, Appl. Environ. Microbiol., 2009, vol. 75, pp. 1589–1596. https://doi.org/10.1128/AEM.02775-08
Somogyi, M., Determination of blood sugar, J. Biol. Chem., 1945, vol. 160, pp. 69–73. https://doi.org/10.1016/S0021-9258(18)43098-0
Tapia, H. and Koshland, D.E., Trehalose is a versatile and long-lived chaperone for desiccation tolerance, Curr. Biol., 2014, vol. 24, pp. 2758–2766. https://doi.org/10.1016/j.cub.2014.10.005
Tereshina, V.M., Memorskaya, A.S., and Kotlova, E.R., The effect of different heat influences on composition of membrane lipids and cytosol carbohydrates in mycelial fungi, Microbiology (Moscow), 2011, vol. 80, pp. 455–460. https://doi.org/10.1134/S0026261711040199
Vaskovsky, V.E., Kostetsky, E.Y., and Vasendin, I.M., A universal reagent for phospholipid analysis, J. Chromatogr. A, 1975, vol. 114, pp. 129–141. https://doi.org/10.1016/S0021-9673(00)85249-8
Weete, J.D., Introduction to fungal lipids, in Fungal Lipid Biochemistry, Kritchevsky, D., Ed., Boston: Springer US, 1974, vol. 1, pp. 3–36. https://doi.org/10.1007/978-1-4684-2829-2_1
Yancey, P.H. and Siebenaller, J.F., Co-evolution of proteins and solutions: protein adaptation versus cytoprotective micromolecules and their roles in marine organisms, J. Exp. Biol., 2015, vol. 218, pp. 1880–1896. https://doi.org/10.1242/jeb.114355
Yancey, P.H., Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses, J. Exp. Biol., 2005, vol. 208, pp. 2819–2830. https://doi.org/10.1242/jeb.01730
Yanutsevich, E.A., Memorskaya, A.S., Groza, N.V., Kochkina, G.A., and Tereshina, V.M., Heat shock response in the thermophilic fungus Rhizomucor miehei, Microbiology (Moscow), 2014, vol. 83, pp. 498–504. https://doi.org/10.1134/S0026261714050282
Yu, R.K., Koerner, T.A.W., Neel Scarsdale, J., Prestegard, J.H., Scarsdale, J.N., and Prestegard, J.H., Elucidation of glycolipid structure by proton nuclear magnetic resonance spectroscopy, Chem. Phys. Lipids, 1986, vol. 42, pp. 27–48. https://doi.org/10.1016/0009-3084(86)90041-1
Funding
The work was supported by the Russian Science Foundation, grant no. 22-74-00040 (https://rscf.ru/en/project/22-74-00040/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by P. Sigalevich
Rights and permissions
About this article
Cite this article
Ianutsevich, E.A., Danilova, O.A., Grum-Grzhimaylo, O.A. et al. Adaptation of the Acidophilic Fungus Sistotrema brinkmannii to the pH Factor. Microbiology 92, 370–378 (2023). https://doi.org/10.1134/S0026261723600210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261723600210