Abstract—
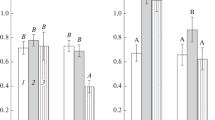
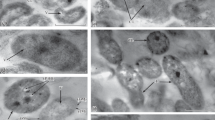
Polyethylene glycol (PEG 6000) was used to establish osmotic stress conditions during growth of the type strain Azospirillum brasilense Sp7 and its spontaneous variants Sp7.4 and Sp7.8, because it causes a stable decrease in the water potential and thus makes it possible to simulate the effect of drought on the bacterial population. While PEG suppressed the motility of azospirilla, it had no effect on the ability of strains Sp7 and Sp7.8 to form biofilms, as well as on the metabolic activity of the biofilms formed in the absence of stress. PEG 6000-caused osmotic stress promoted biofilm formation in Sp7.8. While the biofilms of the Sp7.4 variant were those most sensitive to the negative effect of the water stress, the growth variables of the planktonic culture of this variant under stress conditions exceeded the values for both Sp7 and Sp7.8. In biofilms, strains Sp7, Sp7.4, and Sp7.8 produced polysaccharides and the plant hormone IAA; desiccation-resistant cell forms emerged. The variants Sp7.4 and Sp7.8, similarly to Sp7, formed biofilms during plant root colonization and affected the morphology of the root system of wheat seedlings. Our results show that spontaneous variants of strain Sp7 may be of interest for further research directed at selection of promising Azospirillum strains to enhance the drought resistance of plants.



Similar content being viewed by others
REFERENCES
Ansari, F.A. and Ahmad, I., Fluorescent Pseudomonas FAP2 and Bacillus licheniformis interact positively in biofilm mode enhancing plant growth and photosynthetic attributes, Sci. Rep., 2019, vol. 9, p. 4547.
Ansari, F.A., Jabeen, M., and Ahmad, I., Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant, Int. J. Environ. Sci. Technol., 2021, vol. 18, pp. 3855–3870.
Bashan, Y., Migration of the rhizosphere bacteria Azospirillum brasilense and Pseudomonas fluorescens towards wheat roots in the soil, J. Gen. Microbiol., 1986, vol. 132, pp. 3407‒3414.
Berg, R.H., Tyler, M.E., Novick, N.J., Vasil, V., and Vasil, I.K., Biology of Azospirillum-sugarcane association: enhancement of nitrogenase activity, Appl. Environ. Microbiol., 1980, vol. 39, pp. 642–649.
Bogino, P.C., Oliva, M.M., Sorroche, F.G., and Giordano, W., The role of bacterial biofilms and surface components in plant-bacterial associations, Int. J. Mol. Sci., 2013, vol. 14, pp. 15838–15859.
Chutia, J. and Borah, S.P., Water stress effects on leaf growth and chlorophyll content but not the grain yield in traditional rice (Oryza sativa Linn.) genotypes of Assam, India: II. Protein and proline status in seedlings under PEG induced water stress, Am. J. Plant Sci., 2012, vol. 3, pp. 971–980.
Döbereiner, J. and Day, J.M., Associative symbiosis in tropical grass: Characterization of microorganisms and dinitrogen fixing sites, in Symposium on Nitrogen Fixation, Newton, W.E. and Nijmans, C.J., Eds., Pullman: Washington State Univ., 1976, pp. 518–538.
Fedonenko, Yu.P., Zdorovenko, E.L., Konnova, S.A., Ignatov, V.V., and Shlyakhtin, G.V., A comparison of the lipopolysaccharides and O-specific polysaccharides of Azospirillum brasilense Sp245 and its omegon-Km mutants KM018 and KM252, Microbiology (Moscow), 2004, vol. 73, pp. 143–149.
Fibach-Paldi, S., Burdman, S., and Okon, Y., Key physiological properties contributing to rhizosphere adaptation and plant growth promoting abilities of Azospirillum brasilense, FEMS Microbiol. Lett., 2012, vol. 326, pp. 99–108.
Flemming, H.-C. and Wingender, J., The biofilm matrix, Nat. Rev. Microbiol., 2010, vol. 8, pp. 623–633.
Frolov, A., Bilova, T., Paudel, G., Berger, R., Balcke, G.U., Birkemeyer, C., and Wessjohann, L.A., Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model, J. Plant Physiol., 2017, vol. 208, pp. 70–83.
Fukami, J., Cerezini, P., and Hungria, M., Azospirillum: benefits that go far beyond biological nitrogen fixation, AMB Expr., 2018, vol. 8, pp. 73–85.
Gannesen, A.V., Zdorovenko, E.L., Botchkova, E.A., Hardouin, J., Massier, S., Kopitsyn, D.S., Gorbachev-skii, M.V., Kadykova, A.A., Shashkov, A.S., Zhu-ina, M.V., Netrusov, A.I., Knirel, Y.A., Plakunov, V.K., and Feuilloley, M.G.J., Composition of the biofilm matrix of Cutibacterium acnes acneic strain RT5, Front. Microbiol., 2019, vol. 10, p. 1284. https://doi.org/10.3389/fmicb.2019.01284
Hsiao, T.C., Plant responses to water stress, Ann. Rev. Plant Physiol., 1973, vol. 24, pp. 519–570.
Katsy, E.I. and Petrova, L.P., Genome rearrangements in Azospirillum brasilense Sp7 with the involvement of the plasmid pRhico and the prophage ΦAb-Cd, Russ. J. Genet., 2015, vol. 51, pp. 1165–1171.
Lerner, A., Valverde, A., Castro-Sowinski, S., Lerner, H., Okon, Y., and Burdman, S., Phenotypic variation in Azospirillum brasilense exposed to starvation, Environ. Microbiol. Rep., 2010, vol. 2, pp. 577‒586.
Malinich, E.A. and Bauer, C.E., The plant growth promoting bacterium Azospirillum brasilense is vertically transmitted in Phaseolus vulgaris (common bean), Symbiosis, 2018, vol. 76, pp. 97–108.
Notununu, I., Moleleki, L., Roopnarain, A., and Adeleke, R., Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: a review, Pedosphere, 2022, vol. 32, pp. 90–106.
Oliveira, A.L.M., Canuto, E., Silva, E.E., Veronica, M.R., and Baldani, J.I., Survival of endophytic diazotrophic bacteria in soil under different moisture intensities, Braz. J. Microbiol., 2004, vol. 35, pp. 295–299.
O’Toole, G.A. and Kolter, R., Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis, Mol. Microbiol., 1998, vol. 28, pp. 449–461.
Petrova, L.P., Filip’echeva, Yu.A., Telesheva, E.M., Pylaev, T.E., and Shelud’ko, A.V., Variations in lipopolysaccharide synthesis affect formation of Azospirillum baldaniorum biofilms in planta at elevated copper content, Microbiology (Moscow), 2021, vol. 90, pp. 470–480.
Petrova, L.P., Shelud’ko, A.V., and Katsy, E.I., Plasmid rearrangements and alterations in Azospirillum brasilense biofilm formation, Microbiology (Moscow), 2010, vol. 79, pp. 121–124.
Petrova, L.P., Yevstigneeva, S.S., Borisov, I.V., Shelud’ko, A.V., Burygin, G.L, and Katsy, E.I., Plasmid gene AZOBR_p60126 impacts biosynthesis of lipopolysaccharide II and swarming motility in Azospirillum brasilense Sp245, J. Basic Microbiol., 2020, vol. 60, pp. 613–623.
Sadasivan, L. and Neyra, C.A., Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145, J. Bacteriol., 1987, vol. 169, pp. 1670–1677.
Shelud’ko, A.V., Filip’echeva, Y.A., Telesheva, E.M., Burov, A.M., Evstigneeva, S.S., Burygin, G.L., and Petrova, L.P., Characterization of carbohydrate-containing components of Azospirillum brasilense Sp245 biofilms, Microbiology (Moscow), 2018, vol. 87, pp. 610–620.
Shelud’ko, A.V., Mokeev, D.I., Evstigneeva, S.S., Fi-lip’echeva, Yu.A., Burov, A.M., Petrova, L.P., Ponomareva, E.G., and Katsy, E.I., Cell ultrastructure in biofilms of Azospirillum brasilense, Microbiology (Moscow), 2020, vol. 89, pp. 50–63.
Shelud’ko, A.V., Shirokov, A.A., Sokolova, M.K., Sokolov, O.I., Petrova, L.P., Matora, L.Yu., and Katsy, E.I., Wheat root colonization by Azospirillum brasilense strains with different motility, Microbiology (Moscow), 2010, vol. 79, pp. 688–695.
Tarrand, J.J., Krieg, N.R., and Döbereiner, J., A taxonomic study of the Spirillum lipoferum group with description of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum braslense sp. nov., Can. J. Microbiol., 1978, vol. 24, pp. 967–980.
Volfson, V., Fibach-Paldi, Sh., Paulucci, N.S., Dardanelli, M., Matan, O., Burdman, S., and Okon, Y., Phenotypic variation in Azospirillum brasilense Sp7 does not influence plant growth promotion effects, Soil Biol. Biochem., 2013, vol. 67, pp. 255–262.
Vurukonda, S.S.K.P., Sandhya, V., Shrivastava, M., and Ali, S.K.Z., Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria, Microbiol. Res., 2016, vol. 184, pp. 13–24.
Wang, D., Xu, A., Elmerich, C., and Ma, L.Z., Biofilm formation enables free-living nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions, ISME J., 2017, vol. 11, pp. 1602–1613.
ACKNOWLEDGMENTS
The authors are grateful to the IBPPM Simbioz Center for the Collective Use of Research Equipment in the Field of Physical–Chemical Biology and Nanobiotechnology, IBPPM RAS (Saratov, Russia).
Funding
The work was partially supported by the Russian Foundation for Basic Research, project no. 20-04-00006-a. The work on the assessment of the respiratory activity of the cells was partially supported by Saratov State Medical University, project no. SSMU-2021-001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of inte-rest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by P. Sigalevich
Rights and permissions
About this article
Cite this article
Mokeev, D.I., Volokhina, I.V., Telesheva, E.M. et al. Resistance of Biofilms Formed by the Soil Bacterium Azospirillum brasilense to Osmotic Stress. Microbiology 91, 682–692 (2022). https://doi.org/10.1134/S0026261722601567
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722601567