Abstract—
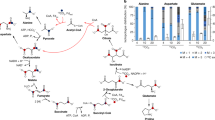
The autotrophic system of CO2 fixation in Chlorobaculum limnaeum strain C consists of two topologically independent enzyme complexes: the reverse tricarboxylic acid cycle (complex I) with acetyl-CoA as the only product, and a noncyclic complex of reactions (complex II). In the reactions catalyzed by the complex II enzymes, acetyl-CoA synthesized in the reverse TCA cycle is used to synthesize all the substrates required for biomass production. Complex I includes two carboxylation reactions catalyzed by 2-oxoglutarate synthase and isocitrate dehydrogenase. Complex II includes two additional carboxylation reactions, which are catalyzed by pyruvate synthase and PEP carboxylase. The enzyme complexes I and II are united into a system of autotrophic CO2 assimilation by citrate synthase, which is not present in the classical variant of the Arnon‒Buchanan cycle. The citrate synthase gene was detected in all studied green sulfur bacteria and may be considered a housekeeping gene.






Similar content being viewed by others
REFERENCES
Alexander, B., Andersen, J.H., Cox, R.P., and Imhoff, J.F., Phylogeny of green sulfur bacteria on the basis of gene sequences of 16S rRNA and of the Fenna‒Matthews‒Olson protein, Arch. Microbiol., 2002, vol. 178, pp. 131‒140. https://doi.org/10.1007/s00203-002-0432-4
Evans, M.C., Buchanan, B.B., and Arnon, D.I., A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium, Proc. Natl. Acad. Sci. USA, 1966, vol. 55, pp. 928‒934. https://doi.org/10.1073/pnas.55.4.928
Hall, T.A., BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT, Nucl. Acids Symp. Ser., 1999, vol. 41, pp. 95‒98.
Hügler, M. and Sievert, S.M., Beyond the Calvin cycle: autotrophic carbon fixation in the ocean, Annu. Rev. Mar. Sci., 2011, vol. 3, pp. 261‒289. https://doi.org/10.1146/annurev-marine-120709-142712
Imhoff, J.F., Phylogenetic taxonomy of the family Chlorobiaceae on the basis of 16S rRNA and fmo (Fenna‒Matthews‒Olson protein) gene sequences, Int. J. Syst. Evol. M-icrobiol., 2003, vol. 53, pp. 941‒951. https://doi.org/10.1099/ijs.0.02403-0
Ivanovsky, R.N., Krasilnikova, E.N., and Fal, Y.I., A pathway of the autotrophic CO2 fixation in Chloroflexus aurantiacus, Arch. Microbiol., 1993, vol. 159, pp. 257‒264. https://doi.org/10.1007/BF00248481
Ivanovsky, R.N., Sintsov, N.V., and Kondratieva, E.N., ATP-linked citrate lyase activity in the green sulfur bacterium Chlorobium limicola forma thiosulfatophilum, Arch. M-icrobiol., 1980, vol. 128, pp. 239‒241. https://doi.org/10.1007/BF00406165
Keppen, O.I., Tourova, T.P., Ivanovsky, R.N., Lebedeva, N.V., Baslerov, R.V., and Berg, I.A., Phylogenetic position of three strains of green sulfur bacteria, Microbiology (Moscow), 2008, vol. 77, pp. 243‒246. https://doi.org/10.1134/S0026261708020203
Larsen, H., On the culture and general physiology of the green sulfur bacteria, J. Bacteriol., 1952, vol. 64, pp. 187‒196. https://doi.org/10.1128/jb.64.2.187-196.1952
Proudfoot, A.T., Bradberry, S.M., and Vale, J.A., Sodium fluoroacetate poisoning, Toxicol. Rev., 2006, vol. 25, pp. 213‒219. https://doi.org/10.2165/00139709-200625040-00002
Saitou, N. and Nei, M., The neighbor-joining method: a new method for reconstructing phylogenetic trees, Mol. B-iol. Evol., 1987, vol. 4, pp. 406‒425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sirevag, R. and Ormerod, J.G., Carbon dioxide-fixation in photosynthetic green sulfur bacteria, Science, 1970, vol. 169, pp. 186‒188. https://doi.org/10.1126/science.169.3941.186
Sweetlove, L.J., and Fernie, A.R., The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation, Nat. Commun., 2018, vol. 9, p. 2136. https://doi.org/10.1038/s41467-018-04543-8
Thompson, J.D., Higgins, D.G., and Gibson, T.J., CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice, Nuc-l. Acids Res., 1994, vol. 22, pp. 4673‒4680. https://doi.org/10.1093/nar/22.22.4673
Trueper, H.G. and Schlegel, H.G., Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii, Antonie van Leeuwenhoek, 1964, vol. 30, pp. 225‒238. https://doi.org/10.1007/bf02046728
Turova, T.P., Kovaleva, O.L., Gorlenko, V.M., and Ivanovskii, R.N., Use of genes of carbon metabolism enzymes as molecular markers of Chlorobi phylum representatives, Microbiology (Moscow), 2013, vol. 83, pp. 784‒793. https://doi.org/10.1134/S0026261714010159
Van de Peer, Y. and De Wachter, R., TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment, Comput. Appl. Biosci., 1994, vol. 10, pp. 569‒570. https://doi.org/10.1093/bioinformatics/10.5.569
Villafranca, J.J. and Platus, E., Fluorocitrate inhibition of aconitase. Reversibility of the inactivation, Biochem. Biophys. Res. Commun., 1973, vol. 55, pp. 1197‒1207. https://doi.org/10.1016/s0006-291x(73)80021-x
ACKNOWLEDGMENTS
The authors are grateful to A.V. Lebedinskii for fruitful discussions during the preparation of the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 20-54-12031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by D. Timchenko
Rights and permissions
About this article
Cite this article
Ivanovsky, R.N., Lebedeva, N.V. & Tourova, T.P. A New Glance on the Mechanism of Autotrophic CO2 Assimilation in Green Sulfur Bacteria. Microbiology 91, 225–234 (2022). https://doi.org/10.1134/S0026261722300026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722300026