Abstract
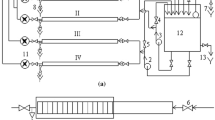
In this study the effect of short-term drying on biofilm-related bacteria was investigated. Biofilm formation was encouraged to develop for nine months in a model water-distribution system. Biofilms were analyzed monthly for enumeration of aerobic and anaerobic heterotrophic bacteria, and sulphate reducing bacteria (SRB) after 6, 24, 48, and 72 hours of exposure to drying. The numbers of live and dead bacteria were directly analyzed by epifluorescence microscopy. In addition, extracellular polysaccharide substances (EPS) extraction, carbohydrate analysis, and scanning electron microscope (SEM) observation were performed. The formation of a brown-colored, thin biofilm layer was observed on the inner surface of polypropylene pipes at the end of the experimental study. SEM micrographs showed that ruptures occurred in the biofilm layer due to effects of drying. The counts of aerobic heterotrophic bacteria and SRB in dried biofilm samples decreased significantly after 6 and 48 hours, respectively. According to 5-cyano-2,3-ditolyl-tetrazolium chloride (CTC) staining results, bacteria can remain viable for up to 72 hours after exposure to drying. The significant increase in the amount of carbohydrate after 48 hours of exposure to drying indicates that bacteria produce EPS as a protective mechanism against drying stress.







Similar content being viewed by others
REFERENCES
Alkan, U., Teksoy, A., and Acar, Ö., Detection of the factors effecting bacterial regrowth in drinking water network, İTÜ dergisi, 2005, vol. 15, pp. 43–55.
Allison, D.G., The biyofilm matrix, Biofouling, 2003, vol. 19, pp. 139–150.
Amel, B.K.N., Amine, B., and Amina, B., Survival of Vibrio fluvialis in seawater under starvation conditions, Microbiol. Res., 2008, vol. 163, pp. 323–328.
Armon, R., Starosvetzky, J., Arbel, T., and Green, M., Survival of Legionella pneumophila and Salmonella typhimurium in biofilm systems, Water Sci. Technol., 1997, vol. 35, pp. 293–300.
Assere, A., Oulahal, N., and Carpentier, B., Comparative evaluation of methods for counting surviving biofilm cells adhering to a polyvinyl chloride surface exposed to chlorine or drying, J. Appl. Microbiol., 2008, vol. 104, pp. 1–11.
Baş, D. and Türetgen, I., The effect of short time drying and temperature change on microbial biofilm formation in a model pipe rig feed with distributed network water, Türk Mikrobiyol. Cem. Derg., 2011, vol. 41, pp. 155–161.
Batte, M., Appenzeller, B.M.R., Grandjean, D., Fass, S., Gauthier, V., Jorand, F., Mathieu, L., Boualam, M., Saby, S., and Block, J.C., Biofilms in drinking water distribution systems, Rev. Environ. Sci. Biotechnol., 2003, vol. 2, pp. 147–168.
Billi, D. and Potts, M., Life and death of dried prokaryotes, Res. Microbiol., 2001, vol. 153, pp. 7–12.
Burke, V., Robinson, J., Gracey, M., Petersen, D., and Partridge, K., Isolation of Aeromonas hydrophila from a metropolitan water supply: seasonal correlation with clinical isolates, Appl. Environ. Microbiol., 1984, vol. 48, pp. 361–366.
Campanac, C., Pineau, L., Payard, A., Baziard-Mouysset, G., and Roques, C., Interactions between biocide cationic agents and bacterial biofilms, Antimicrob. Agents Chemother., 2002, vol. 46, pp. 1469–1474.
Cochran, W.G., Estimation of bacterial densities by means of the “most probable number”, Biometrics, 1950, vol. 6, pp. 105–116.
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., and Smith, F., Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, vol. 28, pp. 350–356.
Ekinci, Ö., Konak, H., and Öztürk, E. A minimum head loss optimization strategy for water distribution networks, Jeodezi, Jeinformasyon ve Arazi Yönetimi Dergisi, 2005, vol. 92, pp. 44–54.
Gagnon, G.A. and Slawson, R.M., An efficient biofilm removal method for bacterial cells exposed to drinking water, J. Microbiol. Methods, 1999, vol. 34, pp. 203–214.
Ilhan-Sungur, E. and Çotuk, A., Microbial corrosion of galvanized steel in a simulated recirculating cooling tower system, Corros. Sci., 2010, vol. 52, pp. 161–171.
Kooij, D.V.D., Lieverloo, J.H.M., Schellart, J., and Hiemstra, P., Maintaining quality without a disinfectant residual, Maintaining Disinfection, 1999, vol. 91, pp. 55–64.
Lee, W. and Characklis, W.G., Corrosion of mild steel under anaerobic biofilm, Corros. Sci., 1993, vol. 49, pp. 186–199.
Momba, M.N.B., Kfir, R., Venter, S.N., and Cloete, T.E., An overview of biofilm formation in distribution systems and its impact on the deterioration of water quality, Water SA, 2000, vol. 26, pp. 59–66.
Panoff, J.M., Thammavongs, B., Guéguen, M., and Boutibonnes, P., Cold stress responses in mesophilic bacteria, Cryobiol., 1998, vol. 36, pp. 75–83.
Peng, J.S., Tsai, W.C., and Chou, C.C. Inactivation and removal of Bacillus cereus by sanitizer and detergent, Int. J. Food Microbiol., 2002, vol. 77, pp. 11–18.
Postgate, J.R. The Sulphate-reducing Bacteria, Cambridge: Cambridge Univ. Press, 1984, 2nd ed.
Potts, M., Desiccation tolerance: a simple process?, Trends Microbiol., 2001, vol. 9, pp. 553–559.
Reasoner, D.J. and Geldrich, E.E. A new medium for the enumeration and subculture of bacteria from potable water, Appl. Environ. Microbiol., 1985, vol. 49, pp. 1–7.
Rodriguez, G.G., Phipps, D., Ishiguro, K., and Ridgway, H.F., Use of a fluorescent redox probe for direct visualization of actively respiring bacteria, Appl. Environ. Microbiol., 1992, vol. 58, pp. 1801–1808.
Signoretto, C., Lleò, M., Tafi, M.C., and Canepari, P., Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state, Appl. Environ. Microbiol., 2000, vol. 66, pp. 1953–1959.
Sutherland, I.W., Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides, Annu. Rev. Microbiol., 1985, vol. 39, pp. 243–270.
Tuovinen, O.H. and Hsu, J.C., Aerobic and anaerobic microorganisms in tubercles of the Columbus, Ohio, water distribution system, Appl. Environ. Microbiol., 1982, vol. 44, pp. 761–764.
Türetgen, I., Ilhan-Sungur, E., and Çotuk, A., Effects of short-time drying on biofilm-associated bacteria, Ann. Microbiol., 2007, vol. 57, pp. 277–280.
Veenendaal, H.R. and Kooij, D., Biofilm Formation Potential of Pipe Materials in Plumbing Systems, Kiwa NV Res. Consult., The Netherlands, Min. Public Hous. Urban Plan. Environ., 1999.
Zhang, X., Bishop, P.L., and Kinkle, B.K., Comparison of extraction methods for quantifying extracellular polymers in biofilms, Water Sci. Technol., 1999, vol. 39, pp. 211–218.
ACKNOWLEDGMENTS
The model system was donated by Dizayn Teknik Plastic Pipes and Fittings Co. This work was supported by Scientific Research Project Coordination Unit of Istanbul University. Project numbers: FDP-2018-28949 and 2803.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Üstüntürk-Onan, M., Hoca, S. & Ilhan-Sungur, E. The Effect of Short-Term Drying on Biofilm Formed in a Model Water Distribution System. Microbiology 87, 857–864 (2018). https://doi.org/10.1134/S0026261718060188
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261718060188