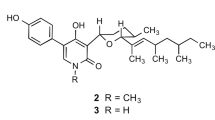
Abstract
A number of pathogenic fungi like Candida, cannot survive upon damage to mitochondrial DNA (mtDNA) while the budding yeast can tolerate the damage therefore we chose Saccharomyces cerevisiae as a model system for this study. Since a number of potent antifungals have originated from various natural sources, we decided to use a triterpenoid and tetraterpenoid in this study as an antifungal agent. Our data clearly indicates that terpenoids play a role in diminishing the mitochondrial content which results in altered level of reactive oxygen species (ROS) and ATP generation. Here, we report that triterpenoid and tetraterpenoid display MIC at 100 and 120 μg /mL respectively against S. cerevisiae. At MIC dose triterpenoid (Lupeol) treated cells showed relatively higher mitochondrial dysfunction as compared to tetraterpenoid, resulting high level of ROS generation in triterpenoid in comparison to tetraterpenoid treated cells. Whereas the ATP level decreases in triterpenoid treated cells while it remains same in tetraterpenoid treated cells. Hence triterpenoid showed more potent antifungal activity as compared to the tetraterpenoid at their MIC by targeting mitochondrial integrity. The outcome of the study is to decipher the mode of action of terpenoids which will be useful in designing of improved antifungal therapies and also accelerate the development of translational applications.
Similar content being viewed by others
References
Amacher, D.E., Drug-associated mitochondrial toxicity and its detection, Curr. Med. Chem., 2005, vol. 12, no. 16, pp. 1829–1839.
Buckingham, J., Dictionary of Natural Products, Web Version 2004, London: Chapman and Hall. Available at: http://www.chemnetbase.com
Cui, Y., Zhao, S., Wu, Z., Dai, P., Zhou, B., Mitochondrial release of the NADH dehydrogenase Ndi1 induces apoptosis in yeast, Mol. Biol. Cell, 2012, vol. 23, pp. 4373–4381.
Fromenty, B. and Pessayre, D., Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity, Pharmacol. Ther., 1995, vol. 67, pp. 101–154.
Ha, H.C. and Snyder, S.H., Poly (ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion, Proc. Natl. Acad. Sci. U. S. A., 1999, vol. 96, pp. 13978–13982.
Jadiya, P. and Nazir, A., Environmental toxicants as extrinsic epigenetic factors for parkinsonism: studies employing transgenic C. elegans model, CNS Neurol. Disord. Drug Targets, 2012, vol. 11, pp. 976–983.
Kim, J.H., Lee, H.O., Cho, Y.J., Kim, J., Chun, J., Choi, J., Lee, Y., and Jung, W.H., A vanillin derivative causes mitochondrial dysfunction and triggers oxidative stress in Cryptococcus neoformans, PLoS One, 2014 doi 10.1371/journal.pone.0089122
Kobayashi, D., Kondo, K., Uehara, N., Otokozawa, S., Tsuji, N., Yagihashi, A., and Watanabe, N., Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect, Antimicrob. Agents Chemother., 2002, vol. 46, pp. 3113–3117.
Kyle, A. R. and Rick, G.S., Isoflavones promote mitochondrial biogenesis, J. Pharmacol. Exp. Therap., 2008, vol. 325, pp. 536–543.
Labbe, G., Pessayre, D., and Fromenty, B., Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies, Fundam. Clin. Pharmacol., 2008, vol. 22, pp. 335–353.
Lakshmi, V., Mahdi, A.A, Ahmad, M.K., Agarwal, S.K., and Srivastava, A.K., Antidiabetic activity of lupeol and lupeol esters in streptozotocin-induced diabetic rats, Bangladesh Pharmaceut. J., 2014, vol. 17, no. 2, pp. 138–146.
Lane, N., Mitochondrial disease: powerhouse of disease, Nature, 2006, vol. 440, pp. 600–602.
Leist, M., Single, B., Castoldi, A.F., Kuhnle, S., and Nicotera, P., Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis, J. Exp. Med., 1997, vol. 185, pp. 1481–1486.
Lesnefsky, E.J., Moghaddas, S., Tandler, B., Kerner, J., and Hoppel, C.L., The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology, IUBMB Life, 2001, vol. 52, pp. 159–164.
Luo, D. Q., Wang, H., Tian, X., Shao, N.J., and Liu, J.K., Antifungal properties of pristimerin and celastrol isolated from Celastrus hypoleucus, Pest Manag. Sci., 2005, vol. 61, pp. 85–90.
Marthanda, M., Subramanyan, M., Hima, M., and Annapurna, J., Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds, Fitoterapia, 2005, vol. 76, pp. 336–339.
Masubuchi, Y., Suda, C., and Horie, T., Involvement of mitochondrial permeability transition in acetaminopheninduced liver injury in mice, J. Hepatol., 2005, vol. 42, pp. 110–116.
Menezes, R.A., Amaral, C., Batista-Nascimento, L., Santos, C., and Ferreira, R.B., Contribution of Yap1 towards Saccharomyces cerevisiae adaptation to arsenic-mediated oxidative stress, Biochem. J., 2008, vol. 414, pp. 301–311.
Nicotera, P. and Leist, M., Energy supply and the shape of death in neurons and lymphoid cells, Cell Death Differ., 1997, vol. 4, pp. 435–442.
Nucci, M. and Perfect, J. R., When primary antifungal therapy fails, Clin. Infect. Dis., 2008, vol. 46, pp. 1426–1433.
Perrone, G.G., Tan, S.X., and Dawes, I.W., Reactive oxygen species and yeast apoptosis, Biochim. Biophys. Acta, 2008, vol. 1783, pp. 1354–1368.
Pfaller, M.A., Diekema, D.J., and Sheehan, D.J., Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing, Clin. Microbiol. Rev., 2006, vol. 19, pp. 435–447.
Rao, A., Zhang, Y., Muend, S., and Rao, R., Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the tor pathway, Antimicrob. Agents Chemother., 2010, vol. 54, no. 12, pp. 5062–5069.
Schapira, A.H., Mitochondrial disease, Lancet, 2006, vol. 368, pp. 70–82.
Sherman, F., Getting started with yeast, Methods Enzymol., 1991, vol. 194, pp. 3–21.
Shingu-Vazquez, M. and Traven, A., Mitochondria and fungal pathogenesis: drug tolerance, virulence, and potential for antifungal therapy, Eukaryot. Cell., 2011, vol. 10, pp. 1376–1383.
Sobel, J.D., Wiesenfeld, H.C., Martens, M., Danna, P., Hooton, T.M., Rompalo, A., Sperling, M., Livengood, C., 3rd, Horowitz, B., Von Thron, J., Edwards, L., Panzer, H., and Chu, TC., Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis, N. Engl. J. Med., 2004, vol. 351, pp. 876–883.
Tsujimoto, Y., Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes, Cell Death Differ., 1997, vol. 4, pp. 429–434.
Varma, J. and Dubey, N.K., Efficacy of essential oils of Caesulia axillaris and Mentha arvensis against some storage pests causing biodeterioration of food commodities, Int. J. Food Microbiol., 2001, vol. 68, pp. 207–210.
Verma, M., Sharma, A., Naidu, S., Bhadra, A.K., Kukreti, R., and Taneja, V., Curcumin prevents formation of polyglutamine aggregates by inhibiting Vps36, a component of the ESCRT-II complex, PLoS One, 2013, doi 10.1371/journal.pone.0042923
White, T.C., Marr, K.A., and Bowden, R.A., Clinical, cellular and molecular factors that contribute to antifungal drug resistance, Clin. Microbiol. Rev., 1998, vol. 11, pp. 382–402.
Zamaraeva, M.V., Sabirov, R.Z., Maeno, E., Ando-Akatsuka, Y., Bessonova, S.V., and Okada, Y., Cells die with increased cytosolic ATP during apoptosis: a bioluminescence study with intracellular luciferase, Cell Death Differentiation, 2005, vol. 12, pp. 1390–1397.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Haque, E., Irfan, S., Kamil, M. et al. Terpenoids with antifungal activity trigger mitochondrial dysfunction in Saccharomyces cerevisiae . Microbiology 85, 436–443 (2016). https://doi.org/10.1134/S0026261716040093
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261716040093