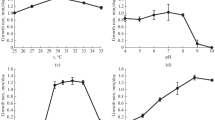
Abstract
We investigated the growth and cell lipid composition of the fungus Mucor hiemalis VKMF-1431 cultivated under aerobic conditions in the presence of the morphogenetic agents itraconazole, exogenous triacylglycerols, and trehalose. The sporangiospores of a 6-day culture were used as inocula. Under these conditions, the fungus produced mycelium; nevertheless, solitary yeastlike cells also developed on the glucose-containing medium and in the presence of itraconazole and sterilized triacylglycerols (sTAGs). No yeastlike growth occurred in the system with trehalose and with unsterilized (native) TAGs (nTAGs). With trehalose and nTAGs in the cultivation medium, the ratio between PEA and PC, the two main types of membrane lipids, was low. This testified to a relatively high PC percentage and, accordingly, a stable structure and a highly functional state of the membranes. Moreover, if the development of the fungus occurred exclusively as mycelium formation, the level of polyunsaturated fatty acids (γ-linolenic and arachidonic acid) increased in the presence of trehalose and that of linoleic acid increased in the presence of nTAGs. These results may suggest that unsaturated fatty acids and membrane lipids are related to the cell wall formation and the implementation of morphogenetic programs in mucorous fungi.
Similar content being viewed by others
References
Nozawa, Y. and Kasai, R., Mechanism of Thermal Adaptation of Membrane Lipids in Tetrahymena pyriformis NT-1. Possible Evidence for Temperature-Mediated Induction of Palmitoyl-CoA Desaturase, Biochim. Biophys. Acta, 1978, vol. 529, no. 1, pp. 54–66.
Rao, T.V., Trivedi, A., and Prasad, P., Phospholipid Enrichment of Saccharomyces cerevisiae and Its Effect on Polyene Sensitivity, Can. J. Microbiol., 1985, vol. 31, no. 4, pp. 322–326.
Rao, T.V., Das, S., and Prasad, P., Effect of Phospholipid Enrichment on Nystatin Action: Differences in Antibiotic Sensitivity between in vivo and in vitro Conditions, Microbios, 1985, vol. 42, no. 169–170, pp. 145–153.
Noverr, M.C., Phare, S.M., Toews, G.B., Coffey, M.J., and Huffnagle, G.B., Pathogenic Yeasts Cryptococccus neoformans and Candida albicans Produce Immunomodulatory Prostaglandins, Infect. Immun., 2001, vol. 69, pp. 2957–2963.
Noverr, M.C., Erb-Downward, J.R., and Huffnagle, G.B., Production of Eicosanoids and Other Oxylipins by Pathogenic Eukaryotic Microbes, Clin. Microbiol. Rev., 2003, vol. 16, no. 3, pp. 517–533.
Noverr, M.C. and Huffnagle, G.B., Regulation of Candida albicans Morphogenesis by Fatty Acid Metabolites, Infect. Immun., 2004, vol. 72, no. 11, pp. 6206–6210.
Klose, J., de Sa, M.M., and Kronstad, J.W., Lipid-Induced Filamentous Growth in Ustilago maydis, Mol. Microbiol., 2004, vol. 52, no. 3, pp. 823–835.
Jeennor, S., Laoteng, K., Tanticharoen, M., and Cheevadhanarak, S., Comparative Fatty Acid Profiling of Mucor rouxii under Different Stress Conditions, FEMS Microbiol. Lett., 2006, vol. 259, no. 1, pp. 60–66.
Macko, V., Inhibitors and Stimulants of Spore Generation and Infection Structure Formation in Fungi, in The Fungal Spore. Morphogenetic Controls, Turian, G. and Holh, H.R., Eds., New York: Academic, 1981, pp. 565–584.
Podila, G.K., Rogers, L.M., and Kolattukudy, P.E., Chemical Signals from Avocado Surface Wax Trigger Germination and Appressorium Formation in Colletotrichum gloeosporioides, Plant Physiol., 1993, vol. 103, pp. 267–272.
Kolattukudy, P.E., Rogers, L.M., Li, D., Hwang, C.S., and Flaishman, M.A., Surface Signaling in Pathogenesis, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, pp. 4080–4087.
Wilson, R.A., Calvo, A.M., Chang, P.K., and Keller, N.P., Characterization of the Aspergillus parasiticus Δ12-Desaturase Gene: A Role for Lipid Metabolism in the Aspergillus-Seed Interaction, Microbiology (UK), 2004, vol. 150, no. 9, pp. 2881–2888.
Orlowski, M., Mucor Dimorphism, Microbiol. Rev., 1991, vol. 55, pp. 234–258.
Illingworth, R.F., Rose, A.H., and Beckett, A., Changes in the Lipid Composition and Fine Structure of Saccharomyces cerevisiae during Ascus Formation, J. Bacteriol., 1973, vol. 113, pp. 373–386.
Calvo, A.M., Gardner, H.W., and Keller, N.P., Genetic Connection between Fatty Acid Metabolism and Sporulation in Aspergillus nidulans, J. Biol. Chem., 2001, vol. 276, pp. 25766–25774.
Mysyakina, I.S. and Funtikova, N.S., The Role of Sterols in Morphogenetic Processes and Dimorphism in Fungi, Microbiology, 2007, vol. 76, no. 1, pp. 1–13.
Mysyakina, I.S. and Feofilova, E.P., The Role of Lipids in the Morphogenetic Processes of Mycelial Fungi, Microbiology, 2011, vol. 80, no. 3, pp. 297–306.
Vanden Bossche, H., Biochemical Targets for Antifungal Azole Derivatives: Hypothesis on the Mode of Action, Curr. Topics Med. Mycol., 1985, vol. 1, pp. 313–351.
Vanden Bossche, H., Importance and Role of Sterols in Fungal Membranes, in Biochemistry of Cell Walls and Membranes in Fungi, Kuhn, P.J., Trinci, A.P.J., Jung, M.J., Goosey, M.W., and Copping, L.G, Eds., Berlin: Springer, 1990, pp. 135–157.
Kerkenaar, A. and Barug, D., Fluorescence Microscope Studies of Ustilago maydis and Penicillium italicum after Treatment with Imazalil or Fenpropimorph, Pestic. Sci., 1984, vol. 15, pp. 199–205.
Marichal, P., Gorrens, J., and Vanden Bossche, H., The Action of Itraconazole and Ketokonazole on Growth and Sterol Synthesis in Aspergillus fumigatus and Aspergillus niger, Sabouraudia, 1985, vol. 23, pp. 13–21.
Barug, D., Samson, R.A., and Kerkenaar, A., Microscopic Studies of Candida albicans and Torulopsis glabrata after in vitro Treatment with Bifonazole, Arzneimittel-Forschung, 1983, vol. 33, pp. 779–782.
Hector, R.F. and Braun, P.C., The Effect of Bifonazole on Chitin Synthesis in Candida albicans, in Recent Trends in the Discovery, Development and Evaluation of Antifungal Agents, Fromtling, R.A. and Prous, J.R., Eds., Barcelona: Science Publ., 1987, pp. 369–382.
Odds, F., Morphogenesis in Candida albicans, CRC Crit. Rev. Microbiol., 1985, vol. 12, pp. 45–93.
Odds, F.C., Cockayne, A., Hayward, J., and Abbott, A.B., Effects of Imidazole and Triazole-Derivative Antifungal Compounds on the Growth and Morphological Development of Candida albicans Hyphae, J. Gen. Microbiol., 1985, vol. 131, pp. 2581–2589.
Nomura, S., Horiuchi, T., Omura, S., and Hata, T., The Action Mechanism of Cerulenin. I. Effect of Cerulenin on Sterol and Fatty Acid Biosynthesis in Yeast, J. Biochem., 1972, vol. 71, no. 5, pp. 783–796.
Greenspan, M.D. and Mackow, R.C., The Effect of Cerulenin on Sterol Biosynthesis in Saccharomyces cerevisiae, Lipids, 1977, vol. 12, no. 9, pp. 729–740.
Ohno, T., Awaya, J., and Omura, S., Inhibition of Sporulation by Cerulenin and Its Reversion by Exogenous Fatty Acids in Saccharomyces cerevisiae, Antimicrob. Agents Chemother., 1976, vol. 9, no. 1, pp. 42–48.
Brambl, R., Wenzler, H., and Josephson, M., Mitochondrial Biogenesis during Fungal Spore Germination: Effects of the Antilipogenic Antibiotic Cerulenin upon Botryodiplodia Spores, J. Bacteriol., 1978, vol. 135, no. 2, pp. 311–317.
Daum, G., Gamerith, G., and Paltauf, F., The Effect of Cerulenin and Exogenous Fatty Acids on Triacylglycerol Accumulation in an Inositol-Deficient Yeast, Saccharomyces carlsbergensis, Biochim. Biophys. Acta, 1979, vol. 573, no. 2, pp. 413–415.
Ito, E., Cihlar, R.L., and Inderlied, C.D., Lipid Synthesis during Morphogenesis in Mucor racemosus, J. Bacteriol., 1982, vol. 152, pp. 880–887.
Sanadi, S., Pandey, R., and Khuller, G.K., Reversal of Cerulenin-Induced Inhibition of Phospholipids and Sterol Synthesis by Exogenous Fatty Acids/Sterols in Epidermophyton floccosum, Biochim. Biophys. Acta, 1987, vol. 921, no. 2, pp. 341–346.
Van Laere, A., Trehalose, Reserve and/or Stress Metabolite?, FEMS Microbiol. Rev., 1989, vol. 63, pp. 201–210.
Wiemken, A., Trehalose in Yeast, Stress Protectant rather Than Reserve Carbohydrate, Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol., 1990, vol. 59, pp. 209–217.
Sampedro, J.G., Guerra, G., Pardo, J.-P., and Uribe, S., Trehalose-Mediated Protection of the Plasma Membrane H+-ATPase from Kluyveromyces lactis during Freeze-Drying and Rehydration, Cryobiol., 1998, vol. 37, pp. 131–138.
D’Amore, T., Crumplen, R., and Stewart, G.G., The Involvement of Trehalose in Yeast Stress Tolerance, J. Ind. Microbiol., 1991, vol. 7, pp. 191–196.
Ribeiro, M.J.S., Leao, L.S.C., Morais, P.B., Rosa, C.A., and Panek, A.D., Trehalose Accumulation by Tropical Yeast Strains Submitted to Stress Conditions, Antonie van Leeuwenhoek, 1999, vol. 75, pp. 245–251.
Lucio, A.K.B., Polizeli, M.L.T.M., Jorge, J.A., and Terenzi, H.F., Stimulation of Hyphal Growth in Anaerobic Cultures of Mucor rouxii by Extracellular Trehalose. Relevance of Cell Wall-Bound Activity of Acid Trehalase for Trehalose Utilization, FEMS Microbiol. Lett., 2000, vol. 182, pp. 9–13.
Funtikova, N.S., Mysyakina, I.S., and Poglazova, M.N., Morphogenesis and Lipid Composition of Mucor Fungi Grown in the Presence of Chloroanilines in Submerged Culture, Microbiology, 1999, vol. 68, no. 4, pp. 406–411.
Mysyakina, I.S. and Funtikova, N.S., Changes in the Lipid Composition of Mucor hiemalis Sporangiospores Related to the Age of the Spore-Forming Culture, Microbiology, 2003, vol. 72, no. 4, pp. 461–465.
Mysyakina, I.S. and Funtikova, N.S., Lipid Composition of the Yeastlike and Mycelial Mucor hiemalis Cells Grown in the Presence of 4-Chloroaniline, Microbiology, 2000, vol. 69, no. 6, pp. 670–675.
Mysyakina, I.S. and Funtikova, N.S., Lipid Composition of the Arthrospores, Yeastlike Cells, and Mycelium of the Fungus Mucor hiemalis, Microbiology, 2001, vol. 70, no. 4, pp. 403–407.
Folch, G., Lees, M., and Sloane-Stanley, G.H., A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem., 1957, vol. 226, no. 1, pp. 497–509.
Bartnicki-Garcia, S., Symposium on Biochemical Bases of Morphogenesis in Fungi. III. Mould-Yeast Dimorphism of Mucor, Bacteriol. Rev., 1963, vol. 27, pp. 293–304.
Sypherd, P.S., Borgia, P., and Pasnokas, J.L., Biochemistry of Dimorphism in the Fungus Mucor, Adv. Microb. Physiol., 1978, vol. 18, pp. 67–104.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.S. Mysiakina, Ya.E. Sergeeva, A.A. Ivashechkin, E.P. Feofilova, 2012, published in Mikrobiologiya, 2012, Vol. 81, No. 6, pp. 726–732.
Rights and permissions
About this article
Cite this article
Mysiakina, I.S., Sergeeva, Y.E., Ivashechkin, A.A. et al. Lipid composition of the mycelium of the fungus Mucor hiemalis cultivated with trehalose, triacylglycerols, and itraconazole. Microbiology 81, 669–675 (2012). https://doi.org/10.1134/S0026261712060094
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261712060094