Abstract
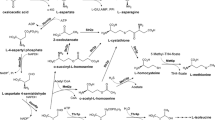
Betaine-type lipids—diacylglyceryltrimethylhomoserines (DGTS)—were revealed in the mycelium of the basidial fungus Flammulina velutipes obtained by surface cultivation on agarized malt extract. DGTS accumulation was shown to occur at the late stages of culture development under deficiency of a complex of nutrients, including nitrogen, phosphorus, potassium, and trace elements. Induction of the synthesis of betaine lipids in F. velutipes occurred against the background of a decreased rate of growth of the vegetative mycelium, formation of monilioid hyphae, and inhibition of fructification. The relationship between DGTS formation and the environmental factors (temperature, illumination) was studied. It was established that the most active DGTS accumulation occurred at 15°C in the dark.
Similar content being viewed by others
References
Brown, A.E. and Elovson, J., Isolation and Characterization of a Novel Lipid, 1(3),2-Diacylglyceryl-(3)-O-4′-(N,N,N-Trimethyl)Homoserine, from Ochromonas danica, Biochemistry, 1974, vol. 13, pp. 3476–3482.
Sato, N., Betaine Lipids, Bot. Mag., 1992, vol. 105, pp. 185–197.
Dembitsky, V.M., Betaine Ether-Linked Glycerolipids: Chemistry and Biology, Prog. Lipid Res., 1996, vol. 35, pp. 1–51.
López-Lara, I.M., Sohlenkamp, C., and Geiger, O., Membrane Lipids in Plant-Associated Bacteria: Their Biosyntheses and Possible Functions, MPMI, 2003, vol. 16, pp. 567–579.
Yamada, T.A. and Nozawa, Y., An Unusual Lipid in the Human Pathogenic Fungus Epidermophyton floccosum, Biochim. Biophys. Acta, 1979, vol. 57, pp. 433–439.
Istokovics, A., Morita, N., Izumi, K., Hoshino, T., Yumoto, I., Sawada, M.T., Ishizaki, K., and Okuyama, H., Neutral Lipids, Phospholipids, and a Betaine Lipid of the Snow Mold Fungus Microdochium nivale, Can. J. Microbiol., 1998, vol. 44, pp. 1051–1059.
Kotlova, E.R. and Popov, E.S., Distribution of Betaine Lipids and Phosphatidylcholines in Ascomycetes, Mikol. Fitopatol., 2005, vol. 39, no. 4, pp. 68–77.
Vaskovsky, V.E., Khotimchenko, S.V., and Benson, A.A., Identification of Diacylglycero-4′-O-(N,N,N-Trimethyl)Homoserine in Mushrooms, Lipids, 1991, vol. 26, pp. 254–256.
Dembitsky, V.M., Shubina, E.E., and Kashin, A.G., Phospholipid and Fatty Acid Compositions of Some Basidiomycetes, Phitochemistry, 1992, vol. 31, pp. 845–849.
Künzler, K. and Eichenberger, W., Betaine Lipids and Zwitterionic Phospholipids in Plants and Fungi, Phytochemistry, 1997, vol. 46, pp. 883–892.
Vaskovsky, V.E., Khotimchenko, S.V., and Boolukh, E.M., Diacylglycerotrimethylhomoserine and Phosphatidylcholine in Mushrooms, Phytochemistry, 1998, vol. 47, pp. 755–760.
Moore, T.S., Du, Z., and Chen, Z., Membrane Lipid Biosynthesis in Chlamydomonas reinhardtii. In vitro Biosynthesis of Diacylglyceryltrimethylhomoserine, Plant Physiol., 2001, vol. 125, pp. 423–429.
Klug, R.M. and Benning, C., Two Enzymes of Diacylglyceryl-O-4-(N,N,N,-Trimethyl)-Homoserine Biosynthesis Are Encoded by btaA and btaB in the Purple Bacterium Rhodobacter sphaeroides, Plant Biol., 2001, vol. 98, pp. 5910–5915.
Riekhof, W.R., Sears, B.B., and Benning, C., Annotation of Genes Involved in Glycerolipid Biosynthesis in Chlamydomonas reinhardtii: Discovery of the Betaine Lipid Synthase BTA1Cr, Eukaryot. Cell, 2005, vol. 4, pp. 242–252.
Khotimchenko, S.V., Klochkova, N.G., and Vaskovsky, V.E., Polar Lipids of Marine Macrophytic Algae as Chemotaxonomic Markers, Biochem. Syst. Ecol., 1990, vol. 18, pp. 93–101.
Benning, C., Huang, Z.H., and Gage, D.A., Accumulation of a Novel Glycolipid and a Betaine Lipid in Cells of Rhodobacter sphaeroides Grown under Phosphate Limitation, Arch. Biochem. Biophys., 1995, vol. 317, pp. 103.
Zavaleta-Pastor, M., Sohlenkamp, C., Gao, J.L., Guan, Z., Zaheer, R., Finan, T.M., Raetz, C.R., López-Lara, I.M., and Geiger, O., Sinorhizobium meliloti Phospholipase C Required for Lipid Remodeling during Phosphorus Limitation, Proc. Natl. Acad. Sci. USA, 2010, vol. 107, pp. 302–307.
Kotlova, E.R. and Sinyutina, N.F., Changes in the Content of Individual Lipid Classes of a Lichen Peltigera aphthosa during Dehydration and Subsequent Rehydration, Russ. J. Plant Physiol., 2005, vol. 52, no. 1, pp. 35–42.
Kotlova, E.R., Senik, S.V., Kücher, T., Shavarda, A.L., Kiyashko, A.A., Psurtseva, N.V., and Zubarev, R.A., Alterations in the Composition of Membrane Glycero- and Sphingolipids in the Course of Flammulina velutipes Surface Culture Development, Microbiology, 2009, vol. 78, no. 2, pp. 193–201.
Bis’ko, N.A., Bukhalo, A.S., Vasser, S.P., Dudka, I.A., Kulesh, M.D., Solomko, E.F., and Shevchenko, S.V., Vysshie s“edobnye bazidiomitsety v poverkhnostnoi i glubinnoi kul’ture (Edible Higher Basidiomycetes in Surface and Submerged Culture), Kiev: Nauk. Dumka, 1983.
Nichols, B.W., Separation of the Lipids of Photosynthetic Tissues: Improvements in Analysis by Thin-Layer Chromatography, Biochem. Biophys. Acta, 1963, vol. 70, pp. 417–425.
Keits, M., Techniques of Lipidology: Isolation, Analysis, and Identification of Lipids, Amsterdam: Elsevier, 1972.
Opekunova, M.G., Arestova, I.Yu., and Elsunova, E.Yu., Metody fiziko-khimicheskogo analiza pochv i rastenii. Metodicheskie ukazaniya (Methods of Physicochemical Analysis of Soil and Plants. Methodic Recommendations), St. Petersburg: Izd-vo SPbGU, 2002.
Stalpers, J.A., Identification of Wood-Inhabiting Aphillophorales in Pure Culture, Stud. Mycol., 1978, no. 16.
Parmeter, J.R., Rhizoctonia solani, Biology and Pathology, Univ. of California Press, 1970.
Matrosova, E.V., Cytological and Immunoenzyme Analysis of the Species of the Genus Agaricus Fr. emend. Karst., Extended Abstract of Cand. Sci. (Biol.) Dissertation, Moscow: Moscow State Univ., 2007.
Sato, N. and Murata, N., Transition of Lipid Phase in Aqueous Dispersions of Diacylglyceryltrimethylhomoserine, Biochem. Biophys. Acta, 1991, vol. 1082, pp. 108–111.
Rozentsvet, O.A., Lipid Composition of Plants as an Indicator of Their Adaptive Capacities for Various Ecological Conditions, Extended Abstract of Doctoral (Biol.) Dissertation, Tolyatti: Inst. Ecol. Volga Basin, 2006.
Kiseleva, M.A., Metabolism of Membrane Lipids in Free-Living and Symbiotic Pseudococcomyxa Green Algae under Phosphorus Limitation, Extended Abstract of Cand. Sci. (Biol.) Dissertation, St.-Petersburg: Botan. Inst., Russ. Acad. Sci., 2008.
Dong, C.-H. and Yao, Y.-J., Nutritional Requirements of Mycelial Growth of Cordyceps sinensis in Submerged Culture, Appl. Microbiol., 2005, vol. 99, pp. 483–492.
Hayes, A.W., Elwanda, P.W., and Patricia, A.K., Environmental and Nutritional Factors Affecting the Production of Rubrotoxin B by Penicillium rubrum Stoll, Appl. Microbiol., 1970, vol. 20, pp. 469–473.
Moustafa, A., Nutrition and the Development of Mushrooms Flavour in Agaricus campestris Mycelium, Appl. Microbiol., 1960, vol. 8, pp. 63–67.
Gao, L. and Liu, X., Effects of Carbon Concentrations and Carbon to Nitrogen Ratios on Sporulation of Two Biological Control Fungi as Determined by Different Culture Methods, Mycopathologia, 2010, vol. 169, pp. 475–481.
Shivrina, A.N., Nizkovskaya, O.P., Falina, N.N., Mattison, N.L., and Efimenko, O.M., Biosinteticheskaya deyatel’nost’ vysshikh gribov (Biosynthetic Activity of Higher Fungi), Leningrad: Nauka, 1969.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.V. Senik, E.R. Kotlova, A.V. Novikov, A.L. Shavarda, N.V. Psurtseva, 2012, published in Mikrobiologiya, 2012, Vol. 81, No. 5, pp. 578–586.
Rights and permissions
About this article
Cite this article
Senik, S.V., Kotlova, E.R., Novikov, A.V. et al. Formation of diacylglyceryltrimethylhomoserines in the surface culture of the basidiomycete Flammulina velutipes . Microbiology 81, 534–541 (2012). https://doi.org/10.1134/S0026261712040145
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261712040145