Abstract
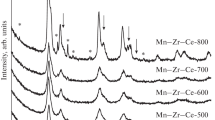
The effect of the calcination temperature and composition of the MnOx–ZrO2 system on its structural characteristics and catalytic properties in the reaction of CO oxidation was studied. According to X-ray diffraction analysis and H2 thermo-programmed reduction data, an increase in the calcination temperature of Mn0.12Zr0.88O2 from 450 to 900°C caused a structural transformation of the system accompanied by the disintegration of solid solution with the release of manganese ions from the structure of ZrO2 and the formation of, initially, highly dispersed MnOx particles and then a crystallized phase of Mn3O4. The dependence of the catalytic activity of MnOx–ZrO2 in the reaction of CO oxidation on the calcination temperature takes an extreme form. A maximum activity was observed after heat treatment at 650–700°C, i.e., at limiting temperatures for the occurrence of a solid solution of manganese ions in the cubic modification of ZrO2. If the manganese content was higher than that in the sample of Mn0.4Zr0.6O2, the phase composition of the system changed: the solid solution phase was supplemented with Mn2O3 and β-Mn3O4 phases. The samples of Mn0.4Zr0.6O2–Mn0.6Zr0.4O2 exhibited a maximum catalytic activity; this was likely due to the presence of the highly dispersed MnOx particles, which were not the solid solution constituents, on their surface in addition to an increase in the dispersity of the solid solution.
Similar content being viewed by others
References
Golodets, G.I., Geterogenno-kataliticheskie reaktsii s uchastiem molekulyarnogo kisloroda (Heterogeneous Catalytic Reactions Involving Molecular Oxygen), Kiev: Naukova Dumka, 1977.
Alvarez-Galvan, M.C., dela Pena O’Shea, V.A., Fierro, J.L.G., and Arias, P.L., Catal. Commun., 2003, vol. 4, p.223.
Liotta, L.F., Appl. Catal. B., 2010, vol. 100, p.403.
Li, W.B., Wang, J.X., and Gong, H., Catal. Today, 2009, vol. 148, p.81.
Kataliticheskie svoistva veshchestv. Spravochnik (Catalytic Properties of Substances: A Handbook), Roiter, V.A., Eds., Kiev: Naukova Dumka, 1968, p. 1462.
Vlasenko, V.M., Mal’chevskii, I.A., Tsetskhladze, D.T., Kuznetsov, V.A., and Vol’fson, V.Ya., Teor. Eksp. Khim., 1984, vol. 20, no. 1, p.49.
Cellier, C., Ruaux, V., Lahousse, C., Grange, P., and Gaigneaux, E.M., Catal. Today, 2006, vol. 117, p.350.
Ramesh, K., Chen, L., Chen, F., Liu, Y., Wang, Z., and Han, Y.-F., Catal. Today, 2008, vol. 131, p.477.
Stobbe, E.R., de Boer, B.A., and Geus, J.W., Catal. Today, 1999, vol. 47, p.161.
Lahousse, C., Bernier, A., Delmon, B., Papaefthimiou, P., Ioannides, T., and Verykios, X., J. Catal., 1998, vol. 178, p.214.
Kapteijn, F., Vanlangeveld, A.D., Moulijn, J.A., Andreini, A., Vuurman, M.A., Turek, A.M., Jehng, J.M., and Wachs, I.E., J. Catal., 1994, vol. 150, p.94.
Imamura, S., Shono, M., Okamoto, N., Hamada, A., and Ishida, S., Appl. Catal., A, 1996, vol. 142, p.279.
Trawczynsky, J., Bielak, B., and Mista, W., Appl. Catal. B, 2005, vol. 55, p.277.
Fernandez Lopez, E., Sanches Ecribano, E., Resini, C., Gallardo-Amores, J.M., and Busca, G., Appl. Catal. B, 2001, vol. 29, p.251.
Chen, H.-R., Shi, J.-L., Zhang, W.-H., Ruan, M.-L., and Yan, D.-S., Microp. Mesopor. Mater., 2001, vol. 47, p.173.
Choudhary, V.R., Uphade, B.S., and Pataskar, S.G., Appl. Catal. A, 2002, vol. 227, p.29.
Gutierrez-Ortiz, J.J., de Rivas, B., Lpez-Forseca, R., Martin, S., and Gonzalez-Velasco, J.R., Chemosphere, 2007, vol. 68, p. 1004.
Zhao, Q., Shih, W.Y., Chang, H.-L., and Shih, W.-H., Ind. Eng. Chem. Res., 2010, vol. 49, p. 1725.
Bulavchenko, O.A., Vinokurov, Z.S., Afonasenko, T.N., Tsyrul’nikov, P.G., Tsybulya, S.V., Saraev, A.A., and Kaichev, V.V., Dalton Trans., 2015, vol. 44, p. 15499.
Dobber, D., Kießling, D., Schmitz, W., and Wendt, G., Appl. Catal. B, 2004, vol. 52, p. 135.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.N. Afonasenko, O.A. Bulavchenko, T.I. Gulyaeva, S.V. Tsybulya, P.G. Tsyrul’nikov, 2018, published in Kinetika i Kataliz, 2018, Vol. 59, No. 1, pp. 127–135.
Rights and permissions
About this article
Cite this article
Afonasenko, T.N., Bulavchenko, O.A., Gulyaeva, T.I. et al. Effect of the Calcination Temperature and Composition of the MnOx–ZrO2 System on Its Structure and Catalytic Properties in a Reaction of Carbon Monoxide Oxidation. Kinet Catal 59, 104–111 (2018). https://doi.org/10.1134/S0023158418010019
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158418010019