Abstract
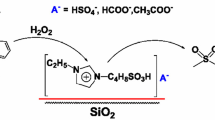
Basic kinetic parameters of the catalytic oxidation of dodecyl mercaptan in kerosene in the presence of silica-immobilized pyridinium or imidazolium chlorocuprate complexes have been determined. The composition of the copper-containing anions and the oxidation kinetics depend on the nature of the ionic liquid and on the method of its synthesis. The compositions developed in this study are usable in the removal of hydrogen sulfide and light mercaptans from the oil stripping gas.
Similar content being viewed by others
References
Ganguly, S.K., Das, G., Kumar, S., Sain, B., and Garg, M.O., Catal. Today, 2012, vol. 198, no. 1, p. 246.
Mazgarov, A.M., Vil’danov, A.F., and Kopylov, Yu.P., Ross. Khim. Zh., 2004, no. 1, p. 67.
Das, G., Sain, B., Kumar, S., and Garg, M.O., Catal. Today, 2009, vol. 141, nos. 1–2, p. 152.
Reza, E.M., Reza, S.A., and Reza, A., Iran. J. Chem. Chem. Eng., 2013, vol. 32, no. 2, p. 71.
Akhmadullina, A.G. and Akhmadullin, R.M., Khim. Tekhnol. Topl. Masel, 2008, no. 6, p. 3.
Bukharkina, T.V. and Verzhichinskaya, S.V., Coke Chem., 2005, no. 11, p. 28.
Menini, L., Pereira, M.C., Ferreira, A.C., Fabris, J.D., and Gusevskaya, E.V., Appl. Catal., A, 2011, vol. 392, nos. 1–2, p. 151.
Javadli, R. and de Klerk, A., Appl. Petrochem. Res., 2012, vol. 1, no. 1, p. 3.
US Patent 8460557, 2013.
Eur. Patent 2105489, 2009.
US Patent 0228528 A1, 2013.
US Patent 2828949, 2012.
US Patent 8460557, 2013.
Tarkhanova, I.G., Gantman, M.G., and Zelikman, V.M., Appl. Catal., A, 2014, vol. 470, no. 1, p. 81.
Tarkhanova, I.G. and Konovalov, V.P., Pet. Chem., 2014, vol. 54, no. 3, p. 218.
Zelikman, V.M., Tarkhanova, I.G., and Khomyakova, E.V., Kinet. Catal., 2012, vol. 53, no. 2, p. 222.
RF Patent 2285917, 2006.
Gilbert, B.C., Silvester, S., and Walton, P.H., J. Chem. Soc., Perkin Trans., 1999, vol. 2, p. 1115.
Tarkhanova, I.G., Gantman, M.G., Chizhov, A.O., and Smirnov, V.V., React. Kinet. Mech. Catal., 2010, vol. 101, p. 267.
Tarkhanova, I.G. and Gantman, M.G., Kinet. Catal., 2011, vol. 52, no. 1, p. 82.
Wang, C., Wesener, S.R., Zhang, H., and Cheng, Y.Q., Chem. Biol., 2009, vol. 16, no. 6, p. 585.
Pomogailo, A.D., Catalysis by Polymer-Immobilized Metal Complexes, Amsterdam Gordon & Breach, 1998.
Bukharkina, T.V., Mazgarov, A.M., Verzhichinskaya, S.V., and Savkin, K.A., Coke Chem., 2005, no. 2, p. 22.
Tyapochkin, E.M. and Kozliak, E.I., J. Mol. Catal. A. Chem., 2005, vol. 242, p. 1.
Bagiyan, G.A., Koroleva, I.K., Soroka, N.V., and Ufimtsev, A.V., Kinet. Catal., 2004, vol. 45, no. 3, p. 372.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.G. Tarkhanova, S.V. Verzhichinskaya, A.K. Buryak, V.M. Zelikman, O.I. Vernaya, R.Z. Sakhabutdinov, R.M. Garifullin, T.V. Bukharkina, L.A. Tyurina, 2017, published in Kinetika i Kataliz, 2017, Vol. 58, No. 4, pp. 384–392.
Rights and permissions
About this article
Cite this article
Tarkhanova, I.G., Verzhichinskaya, S.V., Buryak, A.K. et al. Effect of the composition of the immobilized copper-containing ionic liquid on the dodecyl mercaptan oxidation kinetics. Kinet Catal 58, 362–369 (2017). https://doi.org/10.1134/S002315841704019X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315841704019X