Abstract
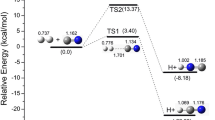
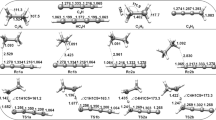
The Δ f H 0298 (NH2CO•) = −8.6 ± 1 kJ/mol and S 0298 = 260.6 ± 5.2 J mol−1 K−1 values have been obtained using the energies of isodesmic reactions within the CBS-Q approximation. Use of the CBS-Q, UMP2, UBHandHLYP, and UB3LYP approaches has afforded the most substantiated entropy value, S 0298 = 258.2 ± 2.8 J mol−1 K−1. The energetics of the NH2CO• ⇄ NH •2 + CO and NH2CO• ⇄ H + NHCO reactions and their rate constants (k 1 and k 2, respectively) have been calculated using the UMP2, UBHandHLYP, and UB3LYP approaches. The rate constant values k 1, ∞ = 8.2 × 1010(T/298)1.18e−115/RT s−1, which were obtained within the UB3LYP approach, are in closest agreement with available experimental data. The constants k 2, ∞ = 4.0 × 107(T/298)1.7e−149/RT s−1, obtained using the UMP2 approach, are best consistent with indirect experimental evidence. The UB3LYP value of the rate constant of the NH •2 + CO reaction at P = 1 atm and T = 304 K (k −1 = 2.2 × 10−18 cm3 molecule−1 s−1) suggests that this reaction should make a significant contribution to the removal of NH •2 from the atmosphere under pre-abiogenesis conditions. The resulting NH2CO• adduct is a fairly stable compound capable of participating in the formation of the chemical composition of the prebiogenic atmosphere. This conclusion is supported by the small rate constant values k 1 = 3.3 × 10−7 s−1 and k 2 = 5.8 × × 10−18 s−1 at P = 1 atm and T = 304 K, which were calculated using the UB3LYP and UMP2 approaches. In addition, the k −2 = 2.8 × 10−21 cm3 molecule−1 s−1 value (P = 1 atm) for the NHCO + H reaction, calculated using the UMP2 approach, indicates that this reaction makes an insignificant contribution to the disappearance of H atoms and to the formation of NH2CO• under the abiogenesis conditions.
Similar content being viewed by others
References
Ehrenfreund, P., Irvine, W., Becker, L., Blank, J., Brucato, J.R., Colangeli, L., Derenne, S., Despois, D., Dutrey, A., Fraaije, H., Lazcano, A., Owen, T., and Robert, F., Rep. Prog. Phys., 2002, vol. 65, p. 1427.
Lal, A.K., Astrophys. Space Sci., 2008, vol. 317, p. 267.
Oparin, A.I., Vozniknovenie zhizni na Zemle (The Origin of Life), Moscow: Biomedizdat, 1936.
Haldane, J.B.S., What Is Life?, New York: Boni & Gaer, 1947.
Miller, S.L., Science, 1953, vol. 117, p. 528.
Urey, H.C., Proc. Natl. Acad. Sci. U.S.A., 1952, vol. 38, p. 351.
Parker, E.T., Cleaves, H.J., Dworkin, J.P., Glavin, D.P., Callahan, M., Aubrey, A., Lazcano, A., and Bada, J.L., PNAS, 2011, vol. 108, p. 5526.
LaRowe, D.E. and Regnier, P., Origins Life Evol. Biosphere, 2008, vol. 38, p. 383.
Oró, J. and Kimball, A.P., Arch. Biochem. Biophys., 1961, vol. 94, p. 217.
Saladino, R., Crestini, C., Costanzo, G., and DiMauro, E., Top. Curr. Chem., 2005, vol. 259, p. 29.
Kobayashi, K., Ogawa, T., and Tonishi, H., Electron. Commun. Jpn., 2008, vol. 91, p. 15.
Khare, B.N., Science, 1971, vol. 173, p. 417.
Yokota, T. and Back, R.A., Int. J. Chem. Kinet., 1973, vol. 5, p. 37.
Back, R.A. and Boden, J.C., Trans. Faraday Soc., 1971, vol. 67, p. 88.
Shapley, W.A. and Bacskay, G.B., J. Phys. Chem. A, 1999, vol. 103, p. 4505.
Nagy, B., Csontos, J., Kallay, M., and Tasi, G., J. Phys. Chem. A, 2010, vol. 114, p. 13213.
Nguyen, M.T., Sengupta, D., Vereecken, L., Peeters, J., and Vanquickenborne, L.G., J. Phys. Chem., 1996, vol. 100, p. 1615.
Golden, D.M., Smith, G.P., McEwen, A.B., Yu, C.-L., Eiteneer, B., Frenklach, M., Yaghjiani, G.L., Ravishankara, A.R., and Tully, F.P., J. Phys. Chem. A, 1998, vol. 102, p. 8598.
Larson, C.W., Stewart, P.H., and Golden, D.M., Int. J. Chem. Kinet., 1988, vol. 20, p. 27.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., and Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision B.03, Pittsburgh, Pa.: Gaussian Inc., 2003.
Mokrushin, V., Bedanov, V., Tsang, W., Zachariah, M.R., and Knyazev, V.D., ChemRate, Version 1.5.10, Gaithersburg, Md.: NIST, 2011.
Senosiain, J.P., Musgrave, C.B., and Golden, D.M., Int. J. Chem. Kinet., 2003, vol. 35, p. 464.
Ruscic, B. and Litorja, M., Chem. Phys. Lett., 2000, vol. 316, p. 45.
Feller, D., Dixon, D.A., and Francisco, J.S., J. Phys. Chem. A, 2003, vol. 107, p. 1604.
Francisco, J.S., Muckerman, J.T., and Yu, H.-G., Acc. Chem. Res., 2010, vol. 43, p. 1519.
Li Jun, Wang Yimin, Jiang Bin, Ma Jianyi, Dawes, R., Xie Daiqian, Bowman, J.M., and Guo Hua, J. Chem. Phys., 2012, vol. 136, p. 041103.
NIST Chemistry WebBook: NIST Standard Reference Database Number 69, Lindstrom, P.J. and Mallard, W.G., Eds., Gaithersburg, Md.: NIST, p. 20899. http://webbook.nist.gov. Accessed June 10, 2014.
Peterson, K.A. and Francisco, J.S., J. Chem. Phys., 2011, vol. 134, no. 084308.
Kasting, J.F., Origins Life Evol. Biosphere, 1990, vol. 20, p. 199.
Kasting, J.F., Zahnle, K.J., and Walker, J.C.G., Precambrian Res., 1983, vol. 20, p. 121.
Feng Tian, Kasting, J.F., and Zahnle, K., Earth Planet. Sci. Lett., 2011, vol. 308, p. 417.
Tsang, W. and Hampson, R.F., J. Phys. Chem. Ref. Data, 1986, vol. 15, p. 1087.
Chao, J., Wilhoit, R.C., and Hall, K.R., Thermochim. Acta, 1980, vol. 41, p. 41.
Bodi, A., Hemberger, P., and Gerber, T., J. Chem. Thermodyn., 2013, vol. 58, p. 292.
Emel’yanenko, V.N., Verevkin, S.P., Varfolomeev, M.A., Turovtsev, V.V., and Orlov, Y.D., J. Chem. Eng. Data, 2011, vol. 56, p. 4183.
Kovács, G., Bencsura, A., Dóbé, S., Bérces, T., and Márt, F., React. Kinet. Catal. Lett., 2005, vol. 86, p. 355.
Da Silva, G. and Bozzelli, J.W., J. Phys. Chem. A, 2006, vol. 110, p. 13058.
De Oliveira, G., Martin, J.M.L., Silwal, I.K.C., and Liebman, J.F., J. Comput. Chem., 2001, vol. 22, p. 1297.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.A. Poskrebyshev, 2015, published in Kinetika i Kataliz, 2015, Vol. 56, No. 3, pp. 251–267.
Rights and permissions
About this article
Cite this article
Poskrebyshev, G.A. Calculating the rate constant for the NH •2 + CO ⇄ NH2CO• ⇄ H + NHCO reactions and thermodynamic properties of NH2CO• . Kinet Catal 56, 245–260 (2015). https://doi.org/10.1134/S0023158415030179
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158415030179