Abstract
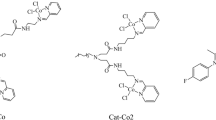
Iron(II) complexes were synthesized with bisiminepyridine ligands of different steric demand. Activation with modified MAO (25 mol% isobutyl groups) generated very active catalysts for propylene oligomerization. These oligomerizations were carried out in liquid propylene in a heat flow calorimeter. The oligomers were separated by preparative gas chromatography and the dimers and trimers analyzed using analytical gas chromatography, 1H-NMR-, and 13C NMR-spectroskopy. By means of the knowledge of the dimer and trimer structure, we were able to establish a mechanistic pathway for propylene insertion and obtained knowledge about the iron alkyl species involved. Analysis of the various dimers formed allowed us to determine the percentage of 1,2-versus 2,1-propylene insertions. Considering the same iron alkyl species with ligands of different steric demand, a change in the probabilities for 1,2-versus 2,1-propylene insertions can be observed. With this knowledge, the catalyst behaviour for ligands of varying steric demand can be predicted. The question of how to produce oligomers versus polymers is one of knowing how to control the ratio of the 1.2-and 2.1-insertion. One method is to alter the steric demand in the ortho position of the ligand. The more bulky the ligand, the more often a 2,1-propylene insertion happens and, therefore, the higher the molecular mass of the oligomers, i.e., polymer is formed. Another important observation is that the formation of α-olefines is favored with a higher steric demand of the catalyst.
Similar content being viewed by others
References
Small, B.L., Brookhart, M., and Bennet, A.M.A., J. Am. Chem. Soc., 1998, vol. 120, p. 4049.
Britovsek, G.J.P., Gibson, V.C., Kimberley, B.S., Maddox, P.J., McTavish, S.J., Solan, G.A., White, A.J.P., and Williams, D.J., Chem. Commun., 1998, p. 848.
Talsi, E.P., Babushkin, D.E., Semikolenova, N.V., Zudin, V.N., and Zakharov, V.A., Kinet. Catal., 2001, vol. 42, p. 147.
Talsi, E.P., Babushkin, D.E., Semikolenova, N.V., Zudin, V.N., Panchenko, V.N., and Zakharov, V.A., Macromol. Chem. Phys., 2001, vol. 202, p. 1816.
Semikolenova, N.V., Zakharov, V.A., Talsi, E.P., Babushkin, D.E., Sobolev, A.P., Echevskaya, L.G., and Khysniyarov, M.M., J. Mol. Catal., 2002, vol. 182, p. 283.
Babik, S.T. and Fink, G., J. Mol. Catal., 2002, vol. 188, p. 245.
Small, B.L. and Brookhart, M., Macromolecules, 1999, vol. 32, p. 2120.
Babik, S.T. and Fink, G., J. Organomet. Chem., 2003, vol. 683, p. 209.
Reardon, D., Conan, F., and Gambrotta, S., J. Am. Chem. Soc., 1999, vol. 121, p. 9318.
Author information
Authors and Affiliations
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Fink, G., Babik, S.T. Propylene bulk phase oligomerization with bisiminepyridine iron catalysts: Mechanistic investigation of 1,2-versus 2,1-propylene insertion. Kinet Catal 47, 198–206 (2006). https://doi.org/10.1134/S0023158406020078
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0023158406020078