Abstract
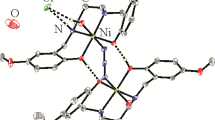
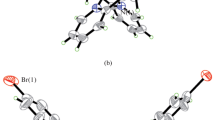
Three zinc(II) complexes, [ZnL(μ1,5-dca)]n·nCH3OH (1), [ZnI2(HL)]·CH3OH (2), and [Zn(NCS)4]·2H2L (3), where L, HL and H2L are the monoanionic, zwitterionic, and monocationic forms of the Schiff base 1-(((2-(pyrrolidin-1-yl)ethyl)imino)methyl)naphthalen-2-ol, dca is dicyanamide, have been successfully synthesized and with their components and structures characterized by CHN elemental analyses, infrared and electronic spectroscopy. The detailed structures are further confirmed by single crystal X-ray determination. The Zn atom in the polymeric complex 1 is in square pyramidal coordination, with the phenolate O, imino N and pyrrolidine N atoms of the Schiff base, and one terminal N atom of dca ligand in the basal plane, and with the other terminal N atom of dca ligand at the apical position. The Zn atom in the mononuclear complex 2 is coordinated by the imino N and phenolate O atoms of the Schiff base, and two iodide ligands, forming tetrahedral coordination. The Zn atom in the mononuclear complex 3 is coordinated by four N atoms from four thiocyanate ligands, forming tetrahedral coordination. The Schiff base and their three metal complexes have been tested for their Jack bean urease activity. As a result, complex 2 has the most activity (IC50 = 3.2±1.1 μmol/L).









Similar content being viewed by others
REFERENCES
A. Ray, C. Nkwonta, P. Forrestal, M. Danaher, K. Richards, T. O′Callaghan, S. Hogan, and E. Cummins. Current knowledge on urease and nitrification inhibitors technology and their safety. Rev. Environ. Health, 2021, 36(4), 477-491. https://doi.org/10.1515/reveh-2020-0088
T. Lan, Y. Huang, X. Song, O. Deng, W. Zhou, L. Luo, X. Tang, J. Zeng, G. Chen, and X. Gao. Biological nitrification inhibitor co-application with urease inhibitor or biochar yield different synergistic interaction effects on NH3 volatilization, N leaching, and N use efficiency in a calcareous soil under rice cropping. Environ. Pollut., 2022, 293, 118499. https://doi.org/10.1016/j.envpol.2021.118499
M. P. Byrne, J. T. Tobin, P. J. Forrestal, M. Danaher, C. G. Nkwonta, K. Richards, E. Cummins, S. A. Hogan, and T. F. O′Callaghan. Urease and nitrification inhibitors - as mitigation tools for greenhouse gas emissions in sustainable dairy systems: A review. Sustainability, 2020, 12(15), 6018. https://doi.org/10.3390/su12156018
A. T. Fiori-Duarte, R. P. Rodrigues, R. R. Kitagawa, and D. F. Kawano. Insights into the design of inhibitors of the urease enzyme - A major target for the treatment of helicobacter pylori infections. Curr. Med. Chem., 2020, 27(23), 3967-3982. https://doi.org/10.2174/0929867326666190301143549
M. Taha, N. H. Ismail, S. Imran, A. Wadood, F. Rahim, and M. Riaz. Synthesis of potent urease inhibitors based on disulfide scaffold and their molecular docking studies. Bioorg. Med. Chem., 2015, 23(22), 7211-7218. https://doi.org/10.1016/j.bmc.2015.10.017
G. I. Pérez-Pérez, C. B. Gower, and M. J. Blaser. Effects of cations on Helicobacter pylori urease activity, release, and stability. Infect. Immun., 1994, 62(1), 299-302. https://doi.org/10.1128/iai.62.1.299-302.1994
R. Mamidala, S. R. S. Bhimathati, and A. Vema. Discovery of novel dihydropyrimidine and hydroxamic acid hybrids as potent Helicobacter pylori urease inhibitors. Bioorg. Chem., 2021, 114, 105010. https://doi.org/10.1016/j.bioorg.2021.105010
S. Iqbal, A. Khan, R. Nazir, S. Kiran, S. Perveen, K. M. Khan, and M. I. Choudhary. Synthesis of β-ketosulfone derivatives as new non-cytotoxic urease inhibitors in vitro. Med. Chem., 2020, 16(2), 244-255. https://doi.org/10.2174/1573406415666190415163309
S. Daud, O.-R. Abid, A. Sardar, B. A. Shah, M. Rafiq, A. Wadood, M. Ghufran, W. Rehman, Zain-ul-Wahab, F. Iftikhar, R. Sultana, H. Daud, and B. Niaz. Design, synthesis, in vitro evaluation, and docking studies on ibuprofen derived 1,3,4-oxadiazole derivatives as dual α-glucosidase and urease inhibitors. Med. Chem. Res., 2022, 31(2), 316-336. https://doi.org/10.1007/s00044-021-02814-6
M. Talebi, E. Hamidian, F. Niasari-Naslaji, S. Rahmani, F. S. Hosseini, S. Boumi, M. N. Montazer, M. Asadi, and M. Amanlou. Synthesis, molecular docking, and biological evaluation of nitroimidazole derivatives as potent urease inhibitors. Med. Chem. Res., 2021, 30(6), 1220-1229. https://doi.org/10.1007/s00044-021-02727-4
M. A. S. Aslam, S. Mahmood, M. Shahid, A. Saeed, and J. Iqbal. Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur. J. Med. Chem., 2011, 46(11), 5473-5479. https://doi.org/10.1016/j.ejmech.2011.09.009
E. Menteşe, M. Emirik, and B. B. Sökmen. Design, molecular docking and synthesis of novel 5,6-dichloro-2-methyl-1H-benzimidazole derivatives as potential urease enzyme inhibitors. Bioorg. Chem., 2019, 86, 151-158. https://doi.org/10.1016/j.bioorg.2019.01.061
Z.-J. Chen, Y.-N. Chen, C.-N. Xu, S.-S. Zhao, Q.-Y. Cao, S.-S. Qian, J. Qin, and H.-L. Zhu. Synthesis, crystal structures, molecular docking, and in vitro biological activities evaluation of transition metal complexes with 4-(3,4-dichlorophenyl) piperazine-1-carboxylic acid. J. Mol. Struct., 2016, 1117, 293-299. https://doi.org/10.1016/j.molstruc.2016.03.084
Z.-P. Xiao, Z.-Y. Peng, J.-J. Dong, R.-C. Deng, X.-D. Wang, H. Ouyang, P. Yang, J. He, Y.-F. Wang, M. Zhu, X.-C. Peng, W.-X. Peng, and H.-L. Zhu. Synthesis, molecular docking and kinetic properties of β-hydroxy-β-phenylpropionyl-hydroxamic acids as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem., 2013, 68, 212-221. https://doi.org/10.1016/j.ejmech.2013.07.047
A. Zianna, E. Vradi, A. G. Hatzidimitriou, S. Kalogiannis, and G. Psomas. Zinc(II) complexes of 3-bromo-5-chloro-salicylaldehyde: characterization and biological activity. Dalton Trans., 2022, 51(46), 17629-17641. https://doi.org/10.1039/d2dt02404g
R. A. C. Souza, V. L. Cunha, J. H. de Souza, C. H. G. Martins, E. de F. Franca, M. Pivatto, J. A. Ellena, L. A. Faustino, A. O. de T. Patrocinio, V. M. Deflon, P. I. da S. Maia, and C. G. Oliveira. Zinc(II) complexes bearing N,N,S ligands: Synthesis, crystal structure, spectroscopic analysis, molecular docking and biological investigations about its antifungal activity. J. Inorg. Biochem., 2022, 237, 111995. https://doi.org/10.1016/j.jinorgbio.2022.111995
A. Zianna, E. Geromichalou, G. Geromichalos, A.-M. Fiotaki, A. G. Hatzidimitriou, S. Kalogiannis, and G. Psomas. Zinc(II) complexes of 3,5-dibromo-salicylaldehyde and α-diimines: Synthesis, characterization and in vitro and in silico biological profile. J. Inorg. Biochem., 2022, 226, 111659. https://doi.org/10.1016/j.jinorgbio.2021.111659
F. Naz, Kanwal, M. Latif, U. Salar, K. M. Khan, M. Al-Rashida, I. Ali, B. Ali, M. Taha, and S. Perveen. 4-Oxycoumarinyl linked acetohydrazide Schiff bases as potent urease inhibitors. Bioorg. Chem., 2020, 105, 104365. https://doi.org/10.1016/j.bioorg.2020.104365
A. Zulfiqar, D. Ahmed, R. Fatima, and S. Yousuf. Green synthesis, urease inhibitory activity and antioxidant potential of 4-bromo-2-(((2′-chloro-4′-nitrophenyl)imino)methyl)phenol Schiff base. J. Mol. Struct., 2020, 1202, 127263. https://doi.org/10.1016/j.molstruc.2019.127263
B. Naureen, G. A. Miana, K. Shahid, M. Asghar, S. Tanveer, and A. Sarwar. Iron(III) and zinc(II) monodentate Schiff base metal complexes: Synthesis, characterisation and biological activities. J. Mol. Struct., 2021, 1231, 129946. https://doi.org/10.1016/j.molstruc.2021.129946
A. A. Osowole, G. A. Kolawole, and O. E. Fagade. Synthesis, characterization and biological studies on unsymmetrical Schiff-base complexes of nickel(II), copper(II) and zinc(II) and adducts with 2,2′-dipyridine and 1,10-phenanthroline. J. Coord. Chem., 2008, 61(7), 1046-1055. https://doi.org/10.1080/00958970701482446
Q. Poladian, O. Şahin, T. Karakurt, B. İlhan-Ceylan, and Y. Kurt. A new zinc(II) complex with N2O2-tetradentate Schiff-base derived from pyridoxal-S-methylthiosemicarbazone: Synthesis, characterization, crystal structure, DFT, molecular docking and antioxidant activity studies. Polyhedron, 2021, 201, 115164. https://doi.org/10.1016/j.poly.2021.115164
A. S. Burlov, V. G. Vlasenko, Y. V. Koshchienko, N. I. Makarova, A. A. Zubenko, Y. D. Drobin, L. N. Fetisov, A. A. Kolodina, Y. V. Zubavichus, A. L. Trigub, S. I. Levchenkov, and D. A. Garnovskii. Synthesis, characterization, luminescent properties and biological activities of zinc complexes with bidentate azomethine Schiff-base ligands. Polyhedron, 2018, 154, 65-76. https://doi.org/10.1016/j.poly.2018.07.034
Y. Tan and Y. Lei. Synthesis and crystal structures of copper, nickel and zinc complexes derived from 2-((2-(pyrrolidin-1-yl)ethylimino)methyl)phenol with antimicrobial activity. Polyhedron, 2023, 231, 116270. https://doi.org/10.1016/j.poly.2022.116270
Bruker. SMART and SAINT. Madison, Wisconsin, USA: Bruker AXS Inc., 2002.
G. M. Sheldrick. SADABS. Göttingen, Germany: University of Göttingen, 1996.
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
W.-J. Mao, P.-C. Lv, L. Shi, H.-Q. Li, and H.-L. Zhu. Synthesis, molecular docking and biological evaluation of metronidazole derivatives as potent Helicobacter pylori urease inhibitors. Bioorg. Med. Chem., 2009, 17(21), 7531-7536. https://doi.org/10.1016/j.bmc.2009.09.018
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, and G. C. Verschoor. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc., Dalton Trans., 1984, (7), 1349-1356. https://doi.org/10.1039/dt9840001349
P. Chakraborty, J. Adhikary, S. Samanta, D. Escudero, A. C. Castro, M. Swart, S. Ghosh, A. Bauzá, A. Frontera, E. Zangrando, and D. Das. Combined experimental and theoretical investigation of ligand and anion controlled complex formation with unprecedented structural features and photoluminescence properties of zinc(II) complexes. Cryst. Growth Des., 2014, 14(8), 4111-4123. https://doi.org/10.1021/cg500717n
G. Marinescu, A. M. Madalan, S. Shova, and M. Andruh. Tetranuclear Zn(II) complexes with compartmental and dicyanamido ligands: Synthesis, structure, and luminescent properties. J. Coord. Chem., 2012, 65(9), 1539-1547. https://doi.org/10.1080/00958972.2012.675435
Y.-N. Guo. Synthesis, crystal structures, and antibacterial activities of Schiff-base zinc(II) complexes [ZnL1Cl2] and [ZnL2I2]·0.5CH3OH. Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2011, 41(8), 987-991. https://doi.org/10.1080/15533174.2011.591332
X.-W. Zhu, Z.-G. Yin, X.-Z. Yang, G.-S. Li, and C.-X. Zhang. {4-Bromo-2-[3-(diethylammonio)propyliminomethyl]phenolato}diiodidozinc(II) methanol solvate. Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65(11), m1293/m1294. https://doi.org/10.1107/s1600536809038446
P. Maiti, A. Khan, T. Chattopadhyay, S. Das, K. Manna, D. Bose, S. Dey, E. Zangrando, and D. Das. Dinuclear zinc(II) complexes with compartmental ligands: Syntheses, structures, and bioactivities as artificial nuclease. J. Coord. Chem., 2011, 64(21), 3817-3831. https://doi.org/10.1080/00958972.2011.631534
O. V. Nesterova, S. R. Petrusenko, V. N. Kokozay, B. W. Skelton, J. K. Bjernemose, and P. R. Raithby. Heterometallic Ni/Zn amine complexes possessing extended 2D and 3D hydrogen-bonded networks prepared from zinc oxide. Inorg. Chim. Acta, 2005, 358(9), 2725-2738. https://doi.org/10.1016/j.ica.2005.02.016
T. Yu, K. Zhang, Y. Zhao, C. Yang, H. Zhang, L. Qian, D. Fan, W. Dong, L. Chen, and Y. Qiu. Synthesis, crystal structure and photoluminescent properties of an aromatic bridged Schiff base ligand and its zinc complex. Inorg. Chim. Acta, 2008, 361(1), 233-240. https://doi.org/10.1016/j.ica.2007.07.012
G. Marinescu, A. M. Madalan, S. Shova, and M. Andruh. Tetranuclear Zn(II) complexes with compartmental and dicyanamido ligands: synthesis, structure, and luminescent properties. J. Coord. Chem., 2012, 65(9), 1539-1547. https://doi.org/10.1080/00958972.2012.675435
S. Basak, S. Sen, S. Banerjee, S. Mitra, G. Rosair, and M. T. G. Rodriguez. Three new pseudohalide bridged dinuclear Zn(II) Schiff base complexes: Synthesis, crystal structures and fluorescence studies. Polyhedron, 2007, 26(17), 5104-5112. https://doi.org/10.1016/j.poly.2007.07.025
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 11, 117920.https://doi.org/10.26902/JSC_id117920
Rights and permissions
About this article
Cite this article
Jiang, J., Liang, P., Deng, Y. et al. Synthesis, Crystal Structures and Urease Inhibition of Zinc(II) Complexes Derived from 1-(((2-(pyrrolidin-1-yl)ethyl)imino)methyl)Naphthalen-2-ol. J Struct Chem 64, 2099–2110 (2023). https://doi.org/10.1134/S0022476623110070
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623110070