Abstract
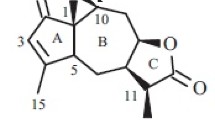
As a result of studying the chemical composition of the Kazakhstan endemic species Tanacetopsis Pjataevae (Tanacetopsis Pjataevae (Kovalevsk.) Karmyscheva), the following guaianolide-type γ-lactones are isolated: arglabin, β-isoepoxyestafiatin, and a new 3-keto-10β-hydroxy-5,7α,4,6β(H)-gvai-1,11(13)-diene-6,12-olide guaianolide. The structure of these compounds is established by X-ray diffraction.




Similar content being viewed by others
REFERENCES
Flora Kazakhstana (Flora of Kazakhstan) / Ed. N. V. Pavlov. Alma-Ata, USSR: Nauka, 1966, Vol. 9. [n Russian]
B. K. Abduazimov, A. I. Yunusov, and G. P. Sidyakin. Sesquiterpene lactones of Tanacetum santolina. Chem. Nat. Compd., 1980, 16(5), 452-454. https://doi.org/10.1007/bf00571036
B. K. Abduazimov, A. I. Yunusov, and G. P. Sidyakin. Components of Tanacetopsis mucronata. Chem. Nat. Compd., 1983, 19(6), 764. https://doi.org/10.1007/bf00575207
M. K. Makhmudov, B. K. Abduazimov, B. Tashkodzhaev, B. T. Ibragimov, and M. R. Yagudaev. Crystal and molecular structure of the sesquiterpene lactone mucrin. Chem. Nat. Compd., 1988, 24(1), 49-53. https://doi.org/10.1007/bf00597573
M. B. Izbosarov, B. K. Abduazimov, A. D. Vdovin, E. L. Kristallovich, and M. P. Yuldashev. Structure of mucroflavone B. Chem. Nat. Compd., 1999, 35(6), 625-627. https://doi.org/10.1007/bf02236287
B. Tashkhodzhaev, B. Kh. Abduazimov, M. B. Izbosarov, I. D. Sham′yanov, and M. Yu. Antipin. 13α-Hydroxymethylenedeacetyllaurenobiolide, a new germacranolide from Tanacetopsis mucronata. Chem. Nat. Compd., 2002, 38(6), 557-560. https://doi.org/10.1023/A:1022682620162
J. J. P. Stewart. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model., 2007, 13(12), 1173-1213. https://doi.org/10.1007/s00894-007-0233-4
APEX2. Software Suite for Crystallographic Programs. Madison, WI, USA: Bruker AXS, 2009.
G. M. Sheldrick. A short history of SHELX. Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64(1), 112-122. https://doi.org/10.1107/s0108767307043930
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
S. M. Adekenov, M. N. Mukhametzhanov, A. D. Kagarlitskii, and A. N. Kupriyanov. Arglabin - A new sesquiterpene lactone from Artemisia glabella. Chem. Nat. Compd., 1982, 18(5), 623/624. https://doi.org/10.1007/bf00575063
R. I. Jalmakhanbetova and S. M. Adekenov. Chemical study of Artemisia filatovae. Chem. Nat. Compd., 2007, 43(3), 347/348. https://doi.org/10.1007/s10600-007-0129-7
K. M. Turdybekov, A. S. Fazylova, and S. M. Adekenov. Stroenie arglabina (The structure of arglabin). Khim. Prir. Soedin, 1998, (Spec. Iss.), 8/9. [In Russian]
S. M. Adekenov and A. D. Kagarlitsriiov. Khimiya seskviterpenovykh laktonov (Chemistry of Sesquiterpene Lactones). Alma-Ata, USSR: Gylym, 1990. [In Russian]
M. Ando and H. Yoshimura. Studies on the syntheses of sesquiterpene lactones. 15. Syntheses of four possible diastereoisomers of Bohlmann′s structure of isoepoxyestafiatin. The stereochemical assignment of isoepoxyestafiatin. J. Org. Chem., 1993, 58(15), 4127-4131. https://doi.org/10.1021/jo00067a056
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc., Perkin Trans. 2, 1987, (12), S1. https://doi.org/10.1039/p298700000s1
J. C. Acosta, F. R. Fronczek, and N. H. Fischer. Micheliolide. Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47(12), 2702-2704. https://doi.org/10.1107/s0108270191007989
R. I. Dzhalmakhanbetova, Y. V. Gatilov, M. M. Shakirov, G. A. Atazhanova, and S. M. Adekenov. Synthesis and molecular structure of halohydrins of the guaianolide ludartin. Chem. Nat. Compd., 2010, 46(2), 222-226. https://doi.org/10.1007/s10600-010-9574-9
Atlas of Steroid Structure / Eds. W. L. Duax and D. A. Norton. New York, USA: IFI/PLENUM, 1975, Vol. 1.
D. L. Ponomarev, K. M. Turdybekov, G. K. Buketova, A. Z. Turmukhambetov, and S. M. Adekenov. Spatial structure of isoepoxyestafiatin. Chem. Nat. Compd., 1997, 33(1), 50/51. https://doi.org/10.1007/bf02273922
P. J. Cox, G. A. Sim, and W. Herz. Sesquiterpenoids. Part XX. X-ray crystallographic determination of the molecular structure of berlandin, a guaianolide epoxide. Comments on the circular dichroism of sesquiterpenoid α-methylene γ-lactones with αβ-unsaturated ester side chains. J. Chem. Soc., Perkin Trans. 2, 1975, (5), 459-463. https://doi.org/10.1039/p29750000459
H. W. Schmalle, K. H. Klaska, and O. Jarchow. Die Kristall- und Molekülstruktur von Arteglasin A, 8α-Acetoxy-3,4-epoxy-4 -guaia-1(10),11(13)-dieno-6α,12-lacton. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1977, 33(7), 2213-2217. https://doi.org/10.1107/s0567740877008024
P. J. Cox. X-ray and molecular mechanics studies on the sesquiterpene lactone eupatocunin-o-bromobenzoate? An unusual case of disorder involving atropisomerism. J. Crystallogr. Spectrosc. Res., 1993, 23(3), 203-208. https://doi.org/10.1007/bf01190048
L. Quijano, T. Rios, R. A. Toscano, and F. R. Fronczek. Structures of 8α-(2′-methylbutyryloxy)-9α-hydroxymontahibisciolide, a new skeletal type of sesquiterpene lactone, and of its precursor 8α-isobutyryloxy-9-oxo-germacra-4E, 1(10)Z-dien-6β,12-olide. J. Chem. Crystallogr., 1996, 26(11), 753-757. https://doi.org/10.1007/bf01664652
A. G. Gonzalez, J. Bermejo, G. M. Massanet, J. M. Amaro, B. Dominguez, and J. Fayos. Hypochaerin, C15H20O3·H2O. Cryst. Struct. Commun., 1977, 6, 373-376.
A. I. Kitaigorodskii. Molekulyarnye kristally (Molecular Crystals). Moscow, USSR: Nauka, 1971. [In Russian]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 9, 115433.https://doi.org/10.26902/JSC_id115433
Rights and permissions
About this article
Cite this article
Turdybekov, K.M., Ivasenko, S.A., Turdybekov, D.M. et al. GUAIANOLIDE-TYPE LACTONES FROM TANACETOPSIS PJATAEVAE: ISOLATION AND SPATIAL STRUCTURE. J Struct Chem 64, 1595–1602 (2023). https://doi.org/10.1134/S0022476623090032
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623090032