Abstract
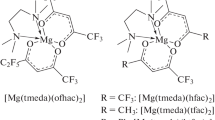
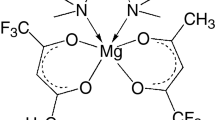
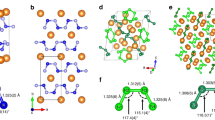
New magnesium complexes [Mg(H2O)2(ofhac)2] 1 and [Mg(tmeda)(ofhac)2] 2 (ofhac = \(\text{C}{{\text{F}}_{\text{3}}}\text{C(O)}\)\(\text{CHC(O)}{{\text{C}}_{\text{2}}}\text{F}_{5}^{-}\), tmeda = N,N,N′,N′-tetramethylethylenediamine) with a 1,1,1,2,2,6,6,6-octafluorohexane-3,5-dionate ligand are prepared. The composition of these compounds is confirmed by elemental analysis and IR spectroscopy, the structure is determined by XRD. The coordination environment of magnesium is distorted octahedral in both complexes. The ofhac ligands are coordinated in the bidentate-cyclic mode; the lengths of Mg–O bonds are similar and vary within 2.033(14)-2.063(18) Å. The aqua ligands in 1 occupy cis positions (d(Mg–O) = 2.0511(18) Å, θ(O–Mg–O) = 85.0(1)°) and participate in the system of O–H…O and O–H…F hydrogen bonds forming a chain packing. The tmeda ligand in 2 performs a chelating function (d(Mg–N) = 2.212(4) Å, θ(N–Mg–N) = 81.0(6)°). The dependence of the structure and the thermal properties of the complexes on the size of the fluorinated substituent (C2F5 instead of CF3) is estimated by comparing the complex with its analogues bearing 1,1,1,5,5,5-hexafluoro-2,4-pentadionate ligands. The crystal structures of [Mg(H2O)2(L)2] are homeotypic; the analogues of [Mg(tmeda)(L)2] crystallize in different space groups. The absence of trans isomers in [Mg(H2O)2(L)2] is confirmed by powder XRD. The thermogravimetry experiments in flowing helium and sublimation tests in vacuum showed that complexes with the ofhac ligand are more volatile. Introducing a C2F5 groups into the ligand also decreases the melting point of the compounds, more significantly for aqua complexes.





Similar content being viewed by others
REFERENCES
V. F. Zinchenko. Solid-phase complex compounds and composites of metal oxides, fluorides, and chalcogenides as materials for interference coatings: a review. Theor. Exp. Chem., 2021, 57(4), 262-271. https://doi.org/10.1007/s11237-021-09694-2
H. K. Raut, V. A. Ganesh, A. S. Nair, and S. Ramakrishna. Anti-reflective coatings: a critical, in-depth review. Energy Environ. Sci., 2011, 4(10), 3779. https://doi.org/10.1039/c1ee01297e
S. Bashir Khan, H. Wu, C. Pan, and Z. Zhang. A mini review: antireflective coatings processing techniques, applications and future perspective. Res. Rev. J. Mater. Sci., 2017, 05(06). https://doi.org/10.4172/2321-6212.1000192
M. Thomann, C. Krause, N. Angrisani, D. Bormann, T. Hassel, H. Windhagen, and A. Meyer-Lindenberg. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res., Part A, 2010, 93(4), 1609-1619. https://doi.org/10.1002/jbm.a.32639
Y. Liu, Y. Zhang, Y.-L. Wang, Y.-Q. Tian, and L.-S. Chen. Research progress on surface protective coatings of biomedical degradable magnesium alloys. J. Alloys Compd., 2021, 885, 161001. https://doi.org/10.1016/j.jallcom.2021.161001
M. E. Fragalà, R. G. Toro, S. Privitera, and G. Malandrino. MOCVD fabrication of magnesium fluoride films: effects of deposition parameters on structure and morphology. Chem. Vap. Deposition, 2011, 17(4-6), 80-87. https://doi.org/10.1002/cvde.201106849
S. Mishra and S. Daniele. Metal–organic derivatives with fluorinated ligands as precursors for inorganic nanomaterials. Chem. Rev., 2015, 115(16), 8379-8448. https://doi.org/10.1021/cr400637c
F. Lo Presti, A. L. Pellegrino, and G. Malandrino. Journey of a molecule from the solid to the gas phase and vice versa: direct estimation of vapor pressure of alkaline-earth metalorganic precursors for atmospheric pressure vapor phase deposition of fluoride films. Dalton Trans., 2022, 51(18), 7352-7362. https://doi.org/10.1039/d2dt00479h
R. Belcher, C. R. Cranley, J. R. Majer, W. I. Stephen, and P. C. Uden. Volatile alkaline earth chelates of fluorinated alkanoylpivalylmethanes. Anal. Chim. Acta, 1972, 60(1), 109-116. https://doi.org/10.1016/s0003-2670(01)81889-4
D. J. Otway and W. S. Rees. Group 2 element β-diketonate complexes: synthetic and structural investigations. Coord. Chem. Rev., 2000, 210(1), 279-328. https://doi.org/10.1016/s0010-8545(00)00360-x
N. V. Kuratieva, E. S. Vikulova, and K. V. Zherikova. Crystal chemistry study of two magnesium complexes with trifluoroacetylacetone. J. Struct. Chem., 2018, 59(1), 131-135. https://doi.org/10.1134/s0022476618010195
M. E. Fragalà, R. G. Toro, P. Rossi, P. Dapporto, and G. Malandrino. Synthesis, characterization, and mass transport properties of a self-generating single-source magnesium precursor for MOCVD of MgF2 films. Chem. Mater., 2009, 21(10), 2062-2069. https://doi.org/10.1021/cm802923w
L. Wang, Y. Yang, J. Ni, C. L. Stern, and T. J. Marks. Synthesis and characterization of low-melting, highly volatile magnesium MOCVD precursors and their implementation in MgO thin film growth. Chem. Mater., 2005, 17(23), 5697-5704. https://doi.org/10.1021/cm0512528
E. S. Vikulova, K. V. Zherikova, I. V. Korolkov, L. N. Zelenina, T. P. Chusova, S. V. Sysoev, N. I. Alferova, N. B. Morozova, and I. K. Igumenov. Thermal properties of mixed-ligand magnesium complexes with beta-diketonates and diamimes as potential MOCVD precursors. J. Therm. Anal. Calorim., 2014, 118(2), 849-856. https://doi.org/10.1007/s10973-014-3997-7
E. S. Vikulova, A. S. Sukhikh, M. A. Mikhaylova, A. A. Nazarova, K. V. Zherikova, and N. B. Morozova. Structure and thermal properties of volatile mixed-ligand magnesium complexes: effect of tert-butyl and phenyl substitutes in a fluorinated β-diketonate. J. Struct. Chem., 2022, 63(8), 1323-1332. https://doi.org/10.1134/s0022476622080133
T. F. Mikhailovskaya, A. G. Makarov, N. Y. Selikhova, A. Y. Makarov, E. A. Pritchina, I. Y. Bagryanskaya, E. V. Vorontsova, I. D. Ivanov, V. D. Tikhova, N. P. Gritsan, Y. G. Slizhov, and A. V. Zibarev. Carbocyclic functionnalization of quinoxalines, their chalcogen congeners 2,1,3-benzothia/selenadiazoles, and related 1,2-diaminobenzenes based on nucleophilic substitution of fluorine. J. Fluor. Chem., 2016, 183, 44-58. https://doi.org/10.1016/j.jfluchem.2016.01.009
V. D. Tikhova, V. P. Fadeeva, O. N. Nikulicheva, T. A. Dobinskaya, and Y. M. Deryabina. Determination of fluorine in organic functional materials. Chem. Sustainable Dev., 2022, 30(6), 640-653. https://doi.org/10.15372/csd2022427
A. A. Coelho. TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr., 2018, 51(1), 210-218. https://doi.org/10.1107/s1600576718000183
T. G. G. Battye, L. Kontogiannis, O. Johnson, H. R. Powell, and A. G. W. Leslie. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr., Sect. D: Biol. Crystallogr., 2011, 67(4), 271-281. https://doi.org/10.1107/s0907444910048675
P. Evans. Scaling and assessment of data quality. Acta Crystallogr., Sect. D: Biol. Crystallogr., 2006, 62(1), 72-82. https://doi.org/10.1107/s0907444905036693
SAINT. Madison, WI, USA: Bruker AXS Inc., 2013.
L. Krause, R. Herbst-Irmer, G. M. Sheldrick, and D. Stalke. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr., 2015, 48(1), 3-10. https://doi.org/10.1107/s1600576714022985
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053273314026370
K. Brandenburg and H. Putz. Diamond - Crystal and Molecular Structure Visualization. Bonn, Germany: Crystal Impact, 1999-2022, https://www.crystalimpact.de/diamond.
S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell, and D. Avnir. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev., 2005, 249(17/18), 1693-1708. https://doi.org/10.1016/j.ccr.2005.03.031
N. P. Kuz′mina, M. V. Ryazanov, S. I. Troyanov, L. I. Martynenko, and I. E. Korsakov. Vacuum sublimation of magnesium and barium hexafluoroacetylacetonate mixtures. Crystal structure of magnesium hexafluoroacetylacetonate dihydrate. Russ. J. Coord. Chem., 1999, 25, 383.
T. Steiner. The hydrogen bond in the solid state. Angew. Chem., Int. Ed., 2002, 41(1), 48-76. https://doi.org/10.1002/1521-3773(20020104)41:1<48::aid-anie48>3.0.co;2-u
B. Morosin. The crystal structure of diaquobis(acetylacetonato)magnesium(II). Acta Crystallogr., 1967, 22(2), 315-320. https://doi.org/10.1107/s0365110x67000556
O. Stryckmans, T. Segato, and P. H. Duvigneaud. Formation of MgO films by ultrasonic spray pyrolysis from β-diketonate. Thin Solid Films, 1996, 283(1/2), 17-25. https://doi.org/10.1016/0040-6090(95)08154-2
Funding
This work was funded by the Russian Science Foundation (project No. 21-73-00252).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 7, 113129.https://doi.org/10.26902/JSC_id113129
Rights and permissions
About this article
Cite this article
Rikhter, E.A., Lee, X., Vikulova, E.S. et al. Mixed-Ligand Precursors for the Preparation of MgF2 Films: Effect of the Fluorinated Substitute on the Structure and Thermal Properties. J Struct Chem 64, 1250–1260 (2023). https://doi.org/10.1134/S0022476623070090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623070090