Abstract
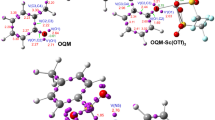
In this work, a MEDT study is carried out on the dehydration of 2-(hydroxy(phenyl)methyl)phenol HPMP to the corresponding o-quinone methide OQM experimentally reported by Tang and coworkers. Both catalyzed and uncatalyzed reactions were studied and it was found that Sc(OTf)3 reduces considerably the activation barriers. The catalyzed reaction takes place initially by the formation of a stable molecular complex between the Sc(OTf)3 Lewis acid and HPMP. The structure of this complex was studied by using MEP map, IGMH and QTAIM analyses and it was found an ionic interaction between the Sc atom and the oxygen atom of HPMP. The electron transfer calculations indicated that the catalyzed reaction is highly polar which is probably responsible for the significant reduction of the activation barriers and consequently performing of the reaction at room temperature under experimental conditions. Finally, the molecular mechanism of the reaction was investigated by ELF analysis and it was concluded that the cleavage of the C4–O5 bond is taken place by polarization of the bonding electron toward the O5 oxygen atom in the first half of the reaction.









Similar content being viewed by others
REFERENCES
L. R. Domingo. Molecular electron density theory: a modern view of reactivity in organic chemistry. Molecules, 2016, 21, 1319. https://doi.org/10.3390/molecules21101319
L. R. Domingo, M. Ríos-Gutiérrez, and P. Pérez. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules, 2016, 21, 748. https://doi.org/10.3390/molecules21060748
R. F. W. Bader. Atoms in molecules. Acc. Chem. Res., 1995, 18, 9-15, https://doi.org/10.1021/ar00109a003
A. D. Becke and K. E. Edgecombe. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys, 1990, 92, 5397-5403. https://doi.org/10.1063/1.458517
E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen, and W. Yang. Revealing noncovalent interactions. J. Am. Chem. Soc., 2010, 132, 6498-6506. https://doi.org/10.1021/ja100936w
L. R. Domingo and N. Acharjee. Molecular Electron Density Theory: A New Theoretical Outlook on Organic Chemistry. Singapore: Bentham and Science, 2020.
M. Soleymani. Mechanistic aspects of the Diels-Alder reaction between (E)-N-benzylidene-2, 2-difluoro-1-phenylethenamine and 2-vinyl pyridine: a molecular electron density theory study. Comput. Theor. Chem., 2022, 113817. https://doi.org/10.1016/j.comptc.2022.113817
M. Soleymani. Coupling of pseudoradical centers in the synthesis of oxazine fused-spiroindoline: a two-stage one-step double cyclization. J. Chem. Sci., 2022, 134, 99. https://doi.org/10.1007/s12039-022-02098-2
M. Soleymani. Regio-, diastereo-and enantioselectivity in the synthesis of CF3-containing spiro [pyrrolidin-3,2′ oxindole] through the organocatalytic [3+2] cycloaddition reaction: A molecular electron density theory study. J. Fluorine Chem., 2020, 109566. https://doi.org/10.1016/j.jfluchem.2020.109566
M. Soleymani, M. Chegeni, and E. Mohammadi. BF3-catalyzed oxa-Diels-Alder reaction of ethyl vinyl sulfide and β-methyl-α-phenylacrolein: a molecular electron density theory study. Monatsh. Chem., 2021, 152, 1209-1221. https://doi.org/10.1007/s00706-021-02841-4
M. Soleymani and S. Emamian. A molecular electron density theory study on the Chichibabin reaction: The origin of regioselectivity. J. Mol. Graphics Modell., 2022, 108240. https://doi.org/10.1016/j.jmgm.2022.108240
A. A. Jaworski and K. A. Scheidt. Emerging roles of in situ generated quinone methides in metal-free catalysis. J. Org. Chem., 2016, 81, 10145-10153. https://doi.org/10.1021/acs.joc.6b01367
N. J. Willis and C. D. Bray. ortho-Quinone methides in natural product synthesis. Chem. Eur. J., 2012, 18, 9160-9173. https://doi.org/10.1002/chem.201200619
M. G. Peter. Chemical modifications of biopolymers by quinones and quinone methides. Angew. Chem., Int. Ed. Engl., 1989, 28, 555-570. https://doi.org/10.1002/anie.198905551
M. S. Singh, A. Nagaraju, N. Anand, and S. Chowdhury. ortho-Quinone methide (o-QM): a highly reactive, ephemeral and versatile intermediate in organic synthesis. RSC Adv., 2014, 4, 55924-55959. https://doi.org/10.1039/C4RA11444B
H. W. Moore. Bioactivation as a model for drug design bioreductive alkylation. Science, 1977, 197, 527-532. https://doi.org/10.1126/science.877572
Y. H. Ma, X. Y. He, Q. Q. Yang, A. Boucherif, and J. Xuan. Recent advances in organocatalytic asymmetric cycloaddition reactions through ortho-quinone methide scaffolds. Asian J. Org. Chem., 2021, 10, 1233-1250. https://doi.org/10.1002/ajoc.202100141
D. V. Osipov, V. A. Osyanin, and Y. N. Klimochkin. ortho-Quinone methides as key intermediates in cascade heterocyclizations. Russ. Chem. Rev., 2017, 86, 625. https://doi.org/10.1070/RCR4679
M. T. M. Martins, F. R. F. Dias, R. S. M. de Moraes, M. F. V. da Silva, K. R. Lucio, K. D′Oliveira Góes, P. A. do Nascimento, A. S. da Silva, V. F. Ferreira, and A. C. Cunha. Multicomponent reactions (MCRs) with o-quinone methides. Chem. Rec., 2022, 22, e202100251. https://doi.org/10.1002/tcr.202100251
R. W. Van De Water and T. R. Pettus. o-Quinone methides: intermediates underdeveloped and underutilized in organic synthesis. Tetrahedron, 2002, 58, 5367-5406. https://doi.org/10.1016/S0040-4020(02)00496-9
M. Uyanik, K. Nishioka, R. Kondo, and K. Ishihara. Chemoselective oxidative generation of ortho-quinone methides and tandem transformations. Nat. Chem., 2020, 12, 353-362. https://doi.org/10.1038/s41557-020-0433-4
M. Spanka and C. Schneider. Phosphoric acid catalyzed aldehyde addition to in situ generated o-quinone methides: an enantio-and diastereoselective entry toward cis-3,4-diaryl dihydrocoumarins. Org. Lett., 2018, 20, 4769-4772. https://doi.org/10.1021/acs.orglett.8b01865
F. Göricke and C. Schneider. Palladium-catalyzed enantioselective addition of chiral metal enolates to in situ generated orth-quinone methides. Angew. Chem. Int. Ed., 2018, 57, 14736-14741. https://doi.org/10.1002/anie.201809692
M. Sun, C. Ma, S. J. Zhou, S. F. Lou, J. Xiao, Y. Jiao, and F. Shi. Catalytic asymmetric (4+3) cyclizations of in situ generated ortho-quinone methides with 2-indolylmethanols. Angew. Chem., 2019, 131, 8795-8800. https://doi.org/10.1002/ange.201901955
P. Batsomboon, W. Phakhodee, S. Ruchirawat, and P. Ploypradith. Generation of ortho-quinone methides by p-TsOH on silica and their hetero-Diels-Alder reactions with styrenes. J. Org. Chem., 2009, 74, 4009-4012. https://doi.org/10.1021/jo900504y
T. N. Purdy, B. S. Moore, and A. L. Lukowski. Harnessing ortho-quinone methides in natural product biosynthesis and biocatalysis. J. Nat. Prod., 2022, 85, 688-701. https://doi.org/10.1021/acs.jnatprod.1c01026
C. D.-T. Nielsen, H. Abas, and A. C. Spivey. Stereoselective reactions of ortho-quinone methide and ortho-quinone methide imines and their utility in natural product synthesis. Synthesis, 2018, 50, 4008-4018. https://doi.org/10.1055/s-0037-1610241
Q. Tan, H. Yu, Y. Luo, F. Chang, X. Liu, Y. Zhou, and X. Feng. Asymmetric catalytic [4+3] cycloaddition of ortho-quinone methides with oxiranes. Chem. Commun., 2021, 57, 3018-3021. https://doi.org/10.1039/D1CC00262G
P.-Z. Dong, B. Qiu, X.-D. An, and J. Xiao. Cascade dearomative [4+2] cycloaddition of indoles with in situ generated ortho-quinone methides: practical access to divergent indoline-fused polycycles. Green Chemistry, 2022, 24, 3772-3777. https://doi.org/10.1039/D2GC00387B
T. Wang, B. Huang, and Y. Q. Wang. Enantioselective synthesis of spiro chroman-isoindolinones via formal (4+2) cycloaddition of in situ-generated ortho-quinone methides with 3-methylene isoindolinones. Adv. Synth. Catal., 2022, 364, 2596-2605. https://doi.org/10.1002/adsc.202200350
C.-Y. Wang, J.-B. Han, L. Wang, and X.-Y. Tang. Lewis acid catalyzed [4+2] cycloaddition of N-tosylhydrazones with ortho-quinone methides. J. Org. Chem., 2019, 84, 14258-14269. https://doi.org/10.1021/acs.joc.9b02040
Y. Zhao and D. G. Truhlar. Comparative DFT study of van der Waals complexes: rare-gas dimers, alkaline-earth dimers, zinc dimer, and zinc-rare-gas dimers. J. Phys. Chem. A, 2006, 110, 5121-5129. https://doi.org/10.1021/jp060231d
C. Gonzalez and H. B. Schlegel. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem., 1990, 94, 5523-5527. https://doi.org/10.1021/j100377a021
A. V. Marenich, C. J. Cramer, and D. G. Truhlar. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B, 2009, 113, 6378-6396. https://doi.org/10.1021/jp810292n
V. Barone, M. Cossi, and J. Tomasi. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem., 1998, 19, 404-417. https://doi.org/10.1002/(sici)1096-987x(199803)19:4<404::aid-jcc3>3.0.co;2-w
M. Cossi, V. Barone, R. Cammi, and J. Tomasi. Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett., 1996, 255, 327-335. https://doi.org/10.1016/0009-2614(96)00349-1
A. E. Reed, R. B. Weinstock, and F. Weinhold. Natural population analysis. J. Chem. Phys, 1985, 83, 735-746. https://doi.org/10.1063/1.449486
T. Lu and F. Chen. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem., 2012, 33, 580-592. https://doi.org/10.1002/jcc.22885
T. Lu and F.-W. Chen. Meaning and functional form of the electron localization function. Acta Phys. - Chim. Sin., 2011, 27, 2786-2792. https://doi.org/10.3866/PKU.WHXB20112786
F. Biegler-König, J. Schönbohm, and D. Bayles. AIM2000. J. Comput. Chem., 2001, 22, 545-559. https://doi.org/10.1002/1096-987x(20010415)22:5<545::aid-jcc1027>3.0.co;2-y
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian09, Revision E.01. Wallingford, CT, USA: Gaussian, Inc., 2009.
L. R. Domingo. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv., 2014, 4, 32415-32428. https://doi.org/10.1039/C4RA04280H
S. Saha and G. N. Sastry. Cooperative or anticooperative: how noncovalent interactions influence each other. J. Phys. Chem. B, 2015, 119, 11121-11135. https://doi.org/10.1021/acs.jpcb.5b03005
D. Cremer and E. Kraka. Chemical bonds without bonding electron density–does the difference electron-density analysis suffice for a description of the chemical bond? Angew. Chem., Int. Ed. Engl., 1984, 23, 627-628. https://doi.org/10.1002/anie.198406271
E. Espinosa, I. Alkorta, J. Elguero, and E. Molins. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys, 2002, 117, 5529-5542. https://doi.org/10.1063/1.1501133
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Text © The Author(s), 2023, published in Zhurnal Strukturnoi Khimii, 2023, Vol. 64, No. 5, 110102.https://doi.org/10.26902/JSC_id110102
Rights and permissions
About this article
Cite this article
Soleymani, M., Goudarzi, M. Sc(OTf)3 Catalyzed Dehydration of 2-(Hydroxy(Phenyl)Methyl)Phenol to ortho-Quinone Methide: a Molecular Electron Density Theory Study. J Struct Chem 64, 859–870 (2023). https://doi.org/10.1134/S0022476623050050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476623050050