Abstract
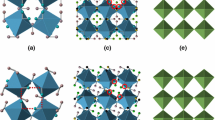
We consider the structural features and symmetry of alkali metal (Li, Na, K, Rb, Cs) and thallium hydroxides crystallizing in LiOH, α-NaOH, β-NaOH, TlOH structure types. The complexities of crystal structures are calculated. The structure of these compounds is based on the alternation of dense layers with strong metal–oxygen bonds and the interlayer space. The common features of hydroxide structures is the location of atoms at square network nodes. Atoms are bound the networks by both true symmetry elements of space groups and additional pseudo-symmetry elements, which indicates the order-disorder (OD) character of hydroxide structures. The arrangement of atoms by the square network pattern allows the consideration of these compounds as a uniform group whose structures retain the tetragonal character of the initial LiOH structure, which is expressed in the similarity of horizontal parameters of their unit cells.











Similar content being viewed by others
REFERENCES
A. K. Ivanov-Schitz and I. V. Murin. Ionika tverdogo tela (Solid-State Ionics). St. Petersburg: St. Petersburg State University, 2000. [In Russian]
O. V. Bushkova, T. V. Yaroslavtseva, and Yu. A. Dobrovolsky. Russ. J. Electrochem., 2017, 53, 677. https://doi.org/10.1134/S1023193517070035
K. Xu. Chem. Rev., 2014, 114, 11503. https://doi.org/10.1021/cr500003w
A. B. Yaroslavtsev. Russ. Chem. Rev., 2016, 84, 1255. https://doi.org/10.1070/RCR4634
D. A. Boryta and A. J. Maas. Ind. Eng. Chem. Process Des. Dev., 1971, 10, 489. https://doi.org/10.1021/i260040a011
G. G. Vurek, D. G. Warnock, and R. Corsey. Anal. Chem., 1975, 47, 765. https://doi.org/10.1021/ac60354a024
L. Schlapbach and A. Züttel. Nature. 2001, 414, 353. https://doi.org/10.1038/35104634
T. K. Mandal and D. H. Gregory. Ann. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2009, 105, 21. https://doi.org/10.1039/b818951j
J. F. Mao, Q. Gu, and D. H. Gregory. Materials. 2015, 8, 2191. https://doi.org/10.3390/ma8052191
T. Ernst. Z. Phys. Chem., Abt. B, 1933, 20, 65. https://doi.org/10.1515/zpch-1933-2006
H. Dachs. Z. Kristallogr. – Cryst. Mater., 1959, 112, 60. https://doi.org/10.1524/zkri.1959.112.jg.60
S. L. Mair. Acta Crystallogr., Sect. A, 1978, 34, 542. https://doi.org/10.1107/S0567739478001151
H. Jacobs, J. Kockelkorn, and T. Tacke. Z. Anorg. Allg. Chem., 1985, 531, 119. https://doi.org/10.1002/zaac.19855311217
H. Stehr. Z. Kristallogr. – Cryst. Mater., 1967, 125, 332. https://doi.org/10.1524/zkri.1967.125.125.332
H.-J. Bleif and H. Dachs. Acta Crystallogr., Sect. A, 1982, 38, 470. https://doi.org/10.1107/S0567739482001028
T. Ernst. Angew. Chem., 1948, 60, 77. https://doi.org/10.1002/ange.19480600308
M. Wörsching and C. Hoch. Z. Naturforsch. B, 2014, 69, 1229. https://doi.org/10.5560/znb.2014-4163
H. Jacobs and B. Harbrecht. Z. Naturforsch. B, 1981, 36, 270. https://doi.org/10.1515/znb-1981-0228
A. Hermann. Phys. Chem. Chem. Phys., 2016, 18, 16527. https://doi.org/10.1039/C6CP03203F
H. P. Beck and G. Lederer. Angew. Chem., 1993, 105, 292. https://doi.org/10.1002/ange.19931050230
H. P. Beck and G. Lederer. J. Chem. Phys., 1993, 98, 7289. https://doi.org/10.1063/1.464721
A. Hermann, N. Ashcroft, and R. Hoffman. J. Chem. Phys., 2014, 141, 024505. https://doi.org/10.1063/1.4886335
O. I. Siidra, S. N. Britvin, S. V. Krivovichev, and W. Depmeier. Z. Anorg. Allg. Chem., 2010, 636, 595. https://doi.org/10.1002/zaac.200900367
S. Krivovichev. Acta Crystallogr., Sect. A, 2012, 68, 393. https://doi.org/10.1107/S0108767312012044
S. V. Krivovichev. Z. Kristallogr., 2018, 233, 155. https://doi.org/10.1515/zkri-2017-2117
V. V. Gurzhiy and J. Plasil. Acta Crystallogr., Sect. B, 2019, 75, 39. https://doi.org/10.1107/S2052520618016098
W. Hornfeck. Acta Crystallogr., Sect. A, 2020, 76, 534. https://doi.org/10.1107/S2053273320006634
I. Csiszár. Entropy, 2008, 10(3), 261. https://doi.org/10.3390/e10030261
A. M. Banaru, S. M. Aksenov, and S. V. Krivovichev. Symmetry, 2021, 13, 1399. https://doi.org/10.3390/sym13081399
A. M. Banaru and S. M. Aksenov. Symmetry, 2022, 14, 220. https://doi.org/10.3390/sym14020220
V. A. Blatov, A. P. Shevchenko, and D. M. Proserpio. Cryst. Growth Des., 2014, 14, 3576. https://doi.org/10.1021/cg500498k
M. OKeeffe, M. A. Peskov, S. J. Ramsden, and O. M. Yaghi. Acc. Chem. Res., 2008, 41, 1782. https://doi.org/10.1021/ar800124u
TopCryst: The Samara Topological Data Center, https://topcryst.com/.
V. A. Blatov, M. OKeeffe, and D. M. Proserpio. CrystEngComm, 2010, 12, 44. https://doi.org/10.1039/B910671E
R. D. Shannon. Acta Crystallogr., Sect. A, 1976, 32, 751. https://doi.org/10.1107/S0567739476001551
D. C. Ghosh and R. Biswas. Int. J. Mol. Sci., 2003, 4, 379. https://doi.org/10.3390/i4060379
Funding
The work was supported by Russian Science Foundation grant No. 20-77-10065 (symmetry features and polytypism - A. M. Banaru and S. M. Aksenov) and the State Assignment for the Vernadsky Institute of Ceochemistry and Analytical Chemistry, Russian Academy of Sciences (calculation of information indices - D.A. Banaru) and the Kola Science Center, Russian Academy of Sciences, No. 122011300125-2 (A. M. Banaru and S. M. Aksenov).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 12, 103704.https://doi.org/10.26902/JSC_id103704
Rights and permissions
About this article
Cite this article
Yamnova, N.A., Banaru, D.A., Banaru, A.M. et al. COMPARATIVE CRYSTAL CHEMISTRY, SYMMETRY FEATURES, AND STRUCTURAL COMPLEXITY OF LiOH, NaOH, RbOH, CsOH, AND TlOH HYDROXIDES. J Struct Chem 63, 2054–2067 (2022). https://doi.org/10.1134/S0022476622120174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622120174