Abstract
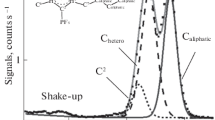
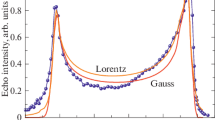
An intricate structure of the X-ray photoelectron spectra of valence and core electrons of potassium ferrate (VI) (K2FeO4) containing Fe6+ ions (3d2) is studied for the first time. Binding energies of core electrons Eb(Fe 3p3/2) = 57.8 eV and Eb(Fe 2p3/2) = 712.1 eV are determined. The mechanism of the appearance of the structure in the Fe 3s spectrum is investigated. The experimental Fe 3s spectrum of Fe6+(3d2) cations in K2FeO4 consists mainly of two lines with a splitting of 2.1 eV. This value is consistent with the results of the theoretical calculation (2.6 eV) for 3d2 (Fe6+) performed by the configuration interaction method.













Similar content being viewed by others
REFERENCES
J. Q. Jiang. J. Hazard. Mater., 2007, 146, 617. https://doi.org/10.1016/j.jhazmat.2007.04.075
S. Wang, Z. Yang, D. Liu, S. Yi, and W. Chi. Electrochem. Commun., 2010, 12, 367. https://doi.org/10.1016/j.elecom.2009.12.036
S. K. Y. D. M. A. and A. M. Russ. J. Inorg. Chem., 2010, 54(6), 942. https://doi.org/10.1134/S0036023610060185
V. V. Nemoshkalenko and V. G. Aleshin. Elektronnaya spektroskopiya kristallov (Electron Spectroscopy of Crystals). Kiev: Naukova Dumka, 1976. [In Russian]
P. S. Bagus, H. J. Freund, T. Minerva, G. Pacchioni, and F. Parmigiani. Chem. Phys. Lett., 1996, 25(1), 90. https://doi.org/10.1016/0009-2614(96)00070-X
A. Yu. Teterin, K. I. Maslakov, Yu. A. Teterin, S. N. Kalmykov, K. E. Ivanov, L. Vukcevic, A. B. Khasanova, and N. S. Shcherbina. Russ. J. Inorg. Chem., 2006, 51(12), 1937. https://doi.org/10.1134/S0036023606120151
Y. A. Teterin, A. V. Sobolev, A. A Belik, Y. S. Glazkova, K. I. , V. G. , . Teterin, K. E. , and I. A. . JETP, 2019, 128, 6. https://doi.org/10.1134/S1063776119050066
V. G. Yarzhemsky, Yu. A. Teterin, I. A. Presnyakov, K. I. Maslakov, A. Yu. Teterin, and K. E. Ivanov. JETP Lett., 2020, 111(8), 422. 10.1134/S0021364020080135
Yu. A. Teterin, A. V. Sobolev, I. A. Presnyakov, K. I. Maslakov, A. Yu. Teterin, I. V. Morozov, I. O. Chernyavskii, K. E. Ivanov, and A. V. Shevelkov. JETP, 2017, 124(2), 251. 10.1134/S1063776117010174
V. G. Yarzhemsky, Yu. A. Teterin, K. I. Maslakov, A. Yu. Teterin, and K. E. Ivanov. JETP Lett., 2021, 114(10), 609. 10.1134/S0021364021220136
L. N. Kramer and M. P. Klein. J. Chem. Phys., 1969, 51, 3618. https://doi.org/10.1063/1.1672562
H. Konno and M. Nagayama. J. Electron. Spectrosc. Relat. Phenom., 1980, 18, 341. 10.1016/0368-2048(80)80021-1
B. Lei, G. Zhou, T. Cheng, and J. Du. Asian J. Chem., 2013, 25(1), 27. 10.14233/ajchem.2013.11685
Handbuch der präparativen anorganischen Chemie / Ed. G. Brauer. Stuttgart: Ferdinand Enke, 1981, Vol. 3, 1653.
S. K. Dedushenko, L. N. Kholodkovskaya, Yu. D. Perfiliev, Yu. M. Kiselev, A. A. Saprykin, P. N. Komozin, and D. G. Lemesheva. J. Alloys Compd., 1997, 262, 78. 10.1016/S0925-8388(97)00332-0
V. I. Goldanskii, A. V. Dolenko, B. G. Egizarov, V. P. Romashko, and A. I. Shamov. Prib. Tekh. Eksp., 1970, 13(4), 101. [In Russian]
D. M. Levin and S. K. Dedushenko. Happy Sloth. Register of computer programs, 2016, 2016660090. www.happysloth.ru
D. A. Shirley. Phys. Rev. B, 1972, 5, 4709. https://doi.org/10.1103/PhysRevB.5.4709
S. K. Dedushenko, Yu. D. Perfiliev, M. G. Goldfeld, and A. I. Tsapin. Hyperfine Interact., 2001, 136/137, 373. 10.1023/A:1020541910373
R. J. Audette, J. W. Quail, W. H. Black, and B. E. Robertson. J. Solid State Chem., 1973, 8, 43. https://doi.org/10.1016/0022-4596(73)90019-4
A. D. Panov. Paket programm obrabotki spektrov SPRO i yazyk programmirovaniya SL (SPRO Spectrum Processing Package and SL Programming Language): Preprint 6019/15. Novosibirsk, Russia: Institute of Automation and Electrometry, 1997.
M. I. Sosulnikov and Yu. A. Teterin. J. Electron. Spectrosc. Relat. Phenom., 1992, 59, 111. 10.1016/0368-2048(92)85002-O
A. P. Grosvenor, B. A. Kobe, M. C. Biesinger, and N. S. McIntyre. Surf. Interface Anal., 2004, 36, 1564. https://doi.org/10.1002/sia.1984
M. Descostes, F. Mercier, N. Thromat, C. Beaucaire, and M. Gautier-Soyer. Appl. Surf. Sci., 2000, 165, 288. https://doi.org/10.1016/S0169-4332(00)00443-8
K. I. Maslakov, Y. A. Teterin, M. V. Ryzhkov, A. J. Popel, A. Y. Teterin, K. E. Ivanov, S. N. Kalmykov, V. G. Petrov, P. K. Petrov, and I. Farnan. Phys. Chem. Chem. Phys., 2018, 20, 16167. https://doi.org/10.1039/c8cp01442f
I. M. Band, Yu. I. Kharitonov, and M. B. Trzhaskovskaya. Atom Data Nucl. Data Tables, 1979, 23, 443. https://doi.org/10.1016/0092-640X(79)90023-8
G. Wendin. Breakdown of the One-Electron Pictures in Photoelectron Spectra: Structure and Bonding, Vol. 45. Berlin, Heidelberg: Springer, 1981. https://doi.org/10.1007/BFb0111503
J. H. Van Vleck. Phys. Rev., 1934, 45(5), 405. https://doi.org/10.1103/PhysRev.45.405
A. G. Kochur, T. M. Ivanova, A. V. Shchukarev, R. V. Linko, A. A. Sidorov, M. A. Kiskin, V. M. Novotortsev, and I. L. Eremenko. J. Electron. Spectrosc. Relat. Phenom., 2010, 180, 21. https://doi.org/10.1016/j.elspec.2010.03.011
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 10, 99693.https://doi.org/10.26902/JSC_id99693
Rights and permissions
About this article
Cite this article
Teterin, Y.A., Perfil’ev, Y.D., Maslakov, K.I. et al. STRUCTURE OF XPS SPECTRA OF K2FeO4. J Struct Chem 63, 1649–1661 (2022). https://doi.org/10.1134/S0022476622100110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622100110