Abstract
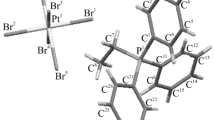
Ionic organyl-triphenylphosphonium 2,4-dinitrobenzenesulfonates [Ph3PR][OSO2C6H3(NO2)2-2,4], R = CH2OMe (1), CH2CN (2), CH2CH = CHCH2PPh3 (3) are prepared by the reaction of organyl-triphenylphosphonium halides with 2,4-dinitrobenzenesulfonic acid in water and are structurally characterized. According to the XRD data, the phosphorus atoms in cations 1-3 have a distorted tetrahedral coordination, the sulfonate anions have a usual geometry with a sulfur atom in a tetrahedral environment. The lengths of P–C bonds vary within 1.742(2)-1.872(2) Å; the CPC bond angles fall within 104.92(6)-113.43(11)°. In the arenesulfonate anions of the complexes, the S–C distances are close to each other (1.743(2)-1.806(3) Å), all the S–O bonds are almost equal (1.4324(15)-1.477(2) Å). The [Ph3PCH2CH=CHCH2PPh3]+ cations of complex 3 contain second-order rotation-reflection axes passing through the centers of the C=C double bonds (point group C2h). The structures of 1, 3 contain short CAr⋯H(Ph) contacts (CH⋯π interactions) (2.829 Å (1), 2.769 Å (3)). The structures of 1-3 are mainly determined by weak intermolecular O⋯H contacts 2.30-2.61 Å (1), 2.23-2.68 Å (2), 2.35-2.69 Å (3).







Similar content being viewed by others
REFERENCES
F. R. Hartley. The Chemistry of Organophosphorus Compounds, Vol. 3: Phosphonium Salts, Ylides and Phosphoranes. John Wiley & Sons, 1993.
C. Cordovilla, C. Bartolome, J. M. Martinez-Ilarduya, and P. Espinet. ACS Catal., 2015, 5, 3040. https://doi.org/10.1021/acscatal.5b00448
C. C. Chong, H. Hirao, and R. Kinjo. Angew. Chem., Int. Ed., 2015, 127, 192. https://doi.org/10.1002/ange.201408760
D. Wang and D. Astruc. Chem. Rev., 2015, 115, 6621. https://doi.org/10.1021/acs.chemrev.5b00203
M. Matsumiya, D. Nomizu, Y. Tsuchida, and Y. Sasaki. Solvent Extr. Ion Exch., 2021, 39, 764. https://doi.org/10.1080/07366299.2021.1889761
G. A. Razuvaev, N. A. Osanova, T. G. Brilkina, T. I. Zinovjeva, and V. V. Sharutin. J. Organomet. Chem., 1975, 99, 93. https://doi.org/10.1016/S0022-328X(00)86365-2
D. Barton and W. D. Ollis. Comprehensive Organic Chemistry, Vol. 2: Phosphorus Compounds. Oxford, UK: Pergamon, 1979.
C. G. Cassity, A. Mirjafari, N. Mobarrez, K. J. Strickland, R. A. OBrien, and J. H. Davis. Chem. Commun., 2013, 49, 7590. https://doi.org/10.1039/c3cc44118k
M. Milenkovic, B. Warzajtis, U. Rychlewska, D. Radanovic, K. Andelkovic, T. Bozic, M. Vujcic, and D. Sladic. Molecules, 2012, 17, 2567. https://doi.org/10.3390/molecules17032567
J. A. Pavlova, Z. Z. Khairullina, A. G. Tereshchenkov, P. A. Nazarov, D. A. Lukianov, I. A. Volynkina, D. A. Skvortsov, G. I. Makarov, E. Abad, S. Y. Murayama, S. Kajiwara, A. Paleskava, A. L. Konevega, Y. N. Antonenko, A. Lyakhovich, I. A. Osterman, A. A. Bogdanov, and N. V. Sumbatyan. Antibiotics, 2021, 10, 489. https://doi.org/10.3390/antibiotics10050489
O. V. Tsepaeva, T. I. Salikhova, L. R. Grigoreva, D. V. Ponomaryov, T. Dang, R. A. Ishkaeva, T. I. Abdullin, A. V. Nemtarev, and V. F. Mironov. Med. Chem. Res., 2021, 30, 925. https://doi.org/10.1007/s00044-020-02674-6
A. V. Artemev, E. A. Pritchina, M. I. Rakhmanova, N. P. Gritsan, I. Yu. Bagryanskaya, S. F. Malysheva, and N. A. Belogorlova. Dalton Trans., 2019, 48, 2328. https://doi.org/10.1039/C8DT04328K
V. V. Sharutin, O. K. Sharutina, N. M. Tarasova, and O. S. Eltsov. Russ. J. Gen. Chem., 2021, 91, 2187. https://doi.org/10.1134/S1070363221110086
A. R. Zykova, V. V. Sharutin, and O. K. Sharutina. Russ. J. Inorg. Chem., 2021, 66, 56. https://doi.org/10.1134/S0036023621010149
V. V. Shatutin, V. S. Senchurin, O. K. Sharutina, and E. A. Boyarkina. Russ. J. Gen. Chem., 2009, 79, 78. https://doi.org/10.1134/S1070363209010125
V. V. Sharutin, O. K. Sharutina, A. V. Rybakova, and Yu. O. Gubanova. Russ J. Gen. Chem., 2018, 88, 1629. https://doi.org/10.1134/S1070363218080133
V. V. Sharutin, N. Mukusheva, and A. V. Urzhumova. Vestn. Yuzhno-Ural. Gos. Univ.: Ser. Khim., 2018, 10, 48. [In Russian] https://doi.org/10.14529/chem180206
V. V. Sharutin, O. K. Sharutina, and Yu. O. Gubanova. Izv. Vyssh. Uchebn. Zaved.: Khim. Khim. Tekhnol., 2019, 62(2), 4. https://doi.org/10.6060/ivkkt.20196202.5823
F. Sodano, B. Rolando, F. Spyrakis, M. Failla, L. Lazzarato, E. Gazzano, C. Riganti, R. Fruttero, A. Gasco, and S. Sortino. ChemMedChem, 2018, 13, 1238. https://doi.org/10.1002/cmdc.201800088
V. F. Mironov, A. V. Nemtarev, O. V. Tsepaeva, M. N. Dimukhametov, I. A. Litvinov, A. D. Voloshina, T. N. Pashirova, E. A. Titov, A. P. Lyubina, S. K. Amerhanova, A. T. Gubaidullin, and D. R. Islamov. Molecules, 2021, 26, 6350. https://doi.org/10.3390/molecules2621635
N. R. Khasiyatullina, A. T. Gubaidullin, A. M. Shinkareva, D. R. Islamov, and V. F. Mironov. Russ. Chem. Bull., Int. Ed., 2020, 69, 2140. https://doi.org/10.1007/s11172-020-3012-3
S. Romanov, A. Aksunova, Y. Bakhtiyarova, M. Shulaeva, O. Pozdeev, S. Egorova, I. Galkina, and V. Galkin. J. Organomet. Chem., 2020, 910, 121130. https://doi.org/10.1016/j.jorganchem.2020.121130
H. Akutsu, K. Masaki, K. Mori, J. Yamada, and S. Nakatsuji. Polyhedron, 2005, 24, 2126. https://doi.org/10.1016/j.poly.2005.03.023
W. I. S. Galpothdeniya, F. R. Fronczek, M. Cong, N. Bhattarai, N. Siraj, and I. M. Warner. J. Mater. Chem. B, 2016, 4, 1414. https://doi.org/10.1039/C5TB02038G
H. Akutsu, J. Yamada, S. Nakatsuji, and S. S. Turner. CrystEngComm, 2009, 11, 2588. https://doi.org/10.1039/b909519e
A. Onoda, Y. Yamada, M. Doi, T. Okamura, and N. Ueyama. Inorg. Chem., 2001, 40, 516. https://doi.org/10.1021/ic0003067
H. Akutsu, K. Ishihara, S. Ito, S. Nishiyama, J. Yamada, S. Nakatsuji, S. S. Turner, and Y. Nakazawa. Polyhedron, 2017, 136, 23. https://doi.org/10.1016/j.poly.2017.02.001
F. Camerel, G. Le Helloco, T. Guizouarn, O. Jeannin, M. Fourmigue, A. Frąckowiak, I. Olejniczak, R. Swietlik, A. Marino, E. Collet, L. Toupet, P. Auban-Senzier, and E. Canadell. Cryst. Growth Des., 2013, 13, 5135. https://doi.org/10.1021/cg401416h
E. G. Ferrer, P. A. M. Williams, and E. E. Castellano. Z. Anorg. Allg. Chem., 2002, 628, 1979. https://doi.org/10.1002/1521-3749(200209)628:9/10<1979::AID-ZAAC1979>3.0.CO;2-V
SMART and SAINT-Plus, Versions 5.0: Data Collection and Processing Software for the SMART System. Madison, Wisconsin, USA: Bruker AXS Inc., 1998.
SHELXTL/PC, Versions 5.10: An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data. Madison, Wisconsin, USA: Bruker AXS Inc., 1998.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339. https://doi.org/10.1107/S0021889808042726
B. N. Tarasevich. IK spektry osnovnykh klassov organicheskikh soedinenii (IR Spectra of the Main Classes of Organic Compounds). Moscow: MGU, 2012. [In Russian]
A. V. Vasilev, E. V. Grinenko, A. O. Schukin, and T. G. Fedulina. Infrakrasnaya spektroskopiya organicheskikh i prirodnykh soedinenii (Infrared Spectroscopy of Organic and Natural Compounds). St. Petersburg: SPbFTU, 2007. [In Russian]
B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, and S. Alvarez. Dalton Trans., 2008, 21, 2832. https://doi.org/10.1039/B801115J
R. Gillespie and I. Hargittai. The VSEPR Model of Geometry. Boston: Allyn &amp; Bacon, 1991.
M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer, and D. G. Truhlar. J. Phys. Chem. A, 2009, 113, 5806. https://doi.org/10.1021/jp8111556
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 10, 99532.https://doi.org/10.26902/JSC_id99532
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K. & Mekhanoshina, E.S. A STUDY OF CRYSTAL STRUCTURES OF ORGANYL-TRIPHENYLPHOSPHONIUM 2,4-DINITROBENZENE SULPHONATES [Ph3PR][OSO2C6H3(NO2)2-2,4], R = CH2OMe, CH2CN, CH2CH = CHCH2PPh3. J Struct Chem 63, 1629–1638 (2022). https://doi.org/10.1134/S0022476622100092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622100092