Abstract
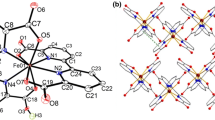
Mononuclear coordination complexes of Mn(II) and Co(II) with ferrocenecarboxylic acid (FcCOOH) with the composition (NBu4)2[M(FcCOO)3(H2O)2)](FcCOO) are prepared. The XRD study showed that the distorted octahedral environment of the metal atom is formed by oxygen atoms of two water molecules and three FcCOO– anions in such a way that two anions are monodentate coordinated and one anion is bidentate-cyclic. The fourth anion is H-bonded with coordinated water molecules. Negatively charged {[M(FcCOO)3(H2O)2)](FcCOO)}– fragments, containing a new type of mononuclear transition metal carboxylate complex, are separated by tetrabutylammonium cations.



Similar content being viewed by others
REFERENCES
C. R. Groom, I. J. Bruno, M. P. Lightfoot, and S. C. Ward. Acta Crystallogr., Sect. B: Struct. Sci. Cryst. Eng. Mater., 2016, 72(2), 171-179. https://doi.org/10.1107/S2052520616003954
E. Fursova, G. Romanenko, R. Sagdeev, and V. Ovcharenko. Polyhedron, 2014, 81, 27-31. https://doi.org/10.1016/j.poly.2014.05.057
V. I. Ovcharenko, E. Y. Fursova, and G. V. Romanenko. Russ. J. Coord. Chem., 2016, 42(9), 584-590. https://doi.org/10.1134/S1070328416090049
E. Y. Fursova, G. V. Romanenko, S. E. Tolstikov, and V. I. Ovcharenko. Russ. Chem. Bull., 2019, 68(9), 1669-1674. https://doi.org/10.1007/s11172-019-2610-4
X.-M. Chen and T. C. W. Mak. Polyhedron, 1991, 10(2), 273-276. https://doi.org/10.1016/S0277-5387(00)81600-9
C. Zheng, R. Shi, X. Jin, L. Dong, and H. Li. Inorg. Chem. Commun., 2015, 58, 74-78. https://doi.org/10.1016/j.inoche.2015.05.022
A. Kundu, S. Saikia, M. Majumder, O. Sengupta, B. Bhattacharya, and G. C. De, S. Ghosh. ACS Omega, 2019, 4(3), 5221-5232. https://doi.org/10.1021/acsomega.9b00101
J. Tong, H.-L. Lu, W.-Q. Sun, and S.-Y. Yu. CrystEngComm, 2020, 22(47), 8166-8170. https://doi.org/10.1039/D0CE00520G
S. S. Shapovalov, A. V. Kolos, A. P. Makhin, I. V. Skabitskii, N. P. Simonenko, and V. V. Minin. J. Struct. Chem., 2019, 60(10), 1648-1654. https://doi.org/10.1134/S002247661910010X
W. Erb, T. Roisnel, and V. Dorcet. Synthesis (Stuttg), 2019, 51(17), 3205-3213. https://doi.org/10.1055/s-0039-1689917.
A. G. Orpen, L. Brammer, F. H. Allen, D. G. Watson, and R. Taylor. In: International Tables for Crystallography. Chester, England: International Union of Crystallography, 2006, 812-896. https://doi.org/10.1107/97809553602060000622
Funding
The study was funded by the Russian Science Foundation, project No. 18-13-00380.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 10, 99181.https://doi.org/10.26902/JSC_id99181
Rights and permissions
About this article
Cite this article
Romanenko, G.V., Tolstikov, S.E., Fursova, E.Y. et al. MONONUCLEAR COMPLEXES OF Mn(II) AND Co(II) WITH FERROCENECARBOXYLIC ACID. J Struct Chem 63, 1579–1583 (2022). https://doi.org/10.1134/S0022476622100043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622100043