Abstract
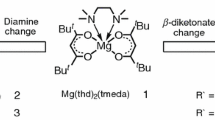
Structures of volatile mixed-ligand complexes of magnesium with N,N,N′,N′-tetramethylethylenediamine (tmeda) and fluorinated β-diketonate ligands L = CF3C(O)CHC(O)R– (R = t-Bu (ptac) and Ph (btfac)) are determined. Complex [Mg(tmeda)(btfac)2] is obtained for the first time and is characterized by elemental analysis and IR spectrometry. Both [Mg(tmeda)(L)2] compounds are molecular complexes, and the symmetry of their crystals is increased due to the presence of an aromatic substituent: space groups Pccn (L = btfac) and P21/n (L = ptac). The magnesium atom occurs in a distorted octahedral environment of three chelate ligands. The bond lengths and chelate angles in both complexes are similar: d(Mg–N) = 2.226(2)-2.245(1) Å, d(Mg–O) = 2.030(2)-2.043(2) Å, θ(N–Mg–N) = 80.97(7)-81.68(10)°, θ(O–Mg–O) = 85.61(9)-85.75(7)°. The Hirshfeld surfaces (CrystalExplorer, Tonto) show a presence of weak intermolecular contacts C–HPh…F in the [Mg(tmeda)(btfac)2] packing (4 contacts per molecule). The qualitative order of volatility decrease for the complexes and their hexafluoroacetylacetonate analogue [Mg(tmeda)(hfac)2] (TGA and vacuum sublimation data) corresponds to the estimated energy increase of [Mg(tmeda)(L)2] crystal lattices (CrystalExplorer, B3LYP/6-31G(d,p)): L = hfac ~ ptac >> btfac.





Similar content being viewed by others
REFERENCES
P. H. Joosten, P. Heller, H. J. Nabben, H. A. van Hal, T. J. Popma, and J. Haisma. Appl. Opt., 1985, 24(16), 2674-2678. https://doi.org/10.1364/AO.24.002674
M. E. Fragala, R. G. Toro, S. Privitera, and G. Malandrino. Chem. Vap. Deposition, 2011, 17(4-6), 80-87. https://doi.org/10.1002/cvde.201106849
M. Mäntymäki, M. Ritala, and M. Leskelä. Coatings, 2018, 8(8), 277. https://doi.org/10.3390/coatings8080277
L. Wang, Y. Yang, J. Ni, C. L. Stern, and T. J. Marks. Chem. Mater., 2005, 17(23), 5697-5704. https://doi.org/10.1021/cm0512528
E. S. Vikulova, K. V. Zherikova, S. V. Sysoev, A. E. Turgambaeva, S. V. Trubin, N. B. Morozova, and I. K. Igumenov. J. Therm. Anal. Calorim., 2019, 137(3), 923-930. https://doi.org/10.1007/s10973-018-07991-y
S. Mishra and S. Daniele. Chem. Rev., 2015, 115(16), 8379-8448. https://doi.org/10.1021/cr400637c
E. S. Vikulova, K. V. Zherikova, I. V. Korolkov, L. N. Zelenina, T. P. Chusova, S. V. Sysoev, N. I. Alferova, N. B. Morozova, and I. K. Igumenov. J. Therm. Anal. Calorim., 2014, 118(2), 849-856. https://doi.org/10.1007/s10973-014-3997-7
H. S. Kim, S. M. George, B. K. Park, S. U. Son, C. G. Kim, and T. M. Chung. Dalton Trans., 2015, 44(5), 2103-2109. https://doi.org/10.1039/C4DT03497J
B. Sedai, M. J. Heeg, and C. H. Winter. J. Organomet. Chem., 2008, 693(23), 3495-3503. https://doi.org/10.1016/j.jorganchem.2008.08.022
E. Pousaneh, T. Rüffer, K. Assim, V. Dzhagan, J. Noll, D. R. Zahn, L. Mertens, M. Mehring, S. E. Schulz, and H. Lang. RSC Adv., 2018, 8, 19668-19678. https://doi.org/10.1039/c8ra01851k
N. V. Kuratieva, E. S. Vikulova, and K. V. Zherikova. J. Struct. Chem., 2018, 59(1), 131-135. https://doi.org/10.1134/S0022476618010195
R. Belcher, C. R. Cranley, J. R. Majer, W. I. Stephen, and P. C. Uden. Anal. Chim. Acta, 1972, 60, 109-116. https://doi.org/10.1016/S0003-2670(01)81889-4
M. E. Fragala, R. G. Toro, P. Rossi, P. Dapporto, and G. Malandrino. Chem. Mater., 2009, 21(10), 2062-2069. https://doi.org/10.1021/cm802923w
I. K. Igumenov, T. V. Basova, and V. R. Belosludov. In: Application of Thermodynamics to Biological and Material Science / Ed. T. Mizutani. Rijeka: InTech, 2011, 521-546. https://doi.org/10.5772/13356
I. Kazadojev, D. J. Otway, and S. D. Elliott. Chem. Vap. Deposition, 2013, 19(4-6), 117-124. https://doi.org/10.1002/cvde.201207025
T. F. Mikhailovskaya, A. G. Makarov, N. Y. Selikhova, A. Y. Makarov, E. A. Pritchina, I. Y. Bagryanskaya, E. V. Vorontsova, I. G. Ivanov, V. D. Tikhova, N. P. Gritsan, Yu. G. Slizhov, and A. V. Zibarev. J. Fluorine Chem., 2016, 183, 44-58. https://doi.org/10.1016/j.jfluchem.2016.01.009
G. M. Sheldrick. Acta Crystallogr., Sect. A., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3-8. https://doi.org/10.1107/S2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339-341. https://doi.org/10.1107/S0021889808042726
S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell, and D. Avnir. Coord. Chem. Rev., 2005, 249(17/18), 1693-1708. https://doi.org/10.1016/j.ccr.2005.03.031
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, and M. A. Spackman. J. Appl. Crystallogr., 2021, 54, 1006-1011. https://doi.org/10.1107/S1600576721002910
D. Jayatilaka and D. J. Grimwood. In: Computational Science - ICCS 2003: Conf. Proc., Melbourne, Australia and St. Petersburg, Russia, June 2-4, 2003 / Eds. P. M. A. Sloot, D. Abramson, A. V. Bogdanov, Y. E. Gorbachev, J. J. Dongarra, and A. Y. Zomaya. Berlin, Heidelberg: Springer, 2003, Vol. 2660, 142-151. https://doi.org/10.1007/3-540-44864-0_15
C. F. Mackenzie, P. R. Spackman, D. Jayatilaka, and M. A. Spackman. IUCrJ, 2017, 4(5), 575-587. https://doi.org/10.1107/S205225251700848X
T. S. Pochekutova, V. K. Khamylov, G. K. Fukin, Y. A. Kurskii, B. I. Petrov, A. S. Shavyrin, and A. V. Arapova. Polyhedron, 2011, 30(12), 1945-1952. https://doi.org/10.1016/j.poly.2011.04.046
S. I. Dorovskikh, E. A. Bykova, N. V. Kuratieva, L. N. Zelenina, Y. V. Shubin, N. B. Morozova, and I. K. Igumenov. J. Organomet. Chem., 2012, 698, 22-27. https://doi.org/10.1016/j.jorganchem.2011.10.020
M. Klotzsche, D. Barreca, L. Bigiani, R. Seraglia, A. Gasparotto, L. Vanin, C. Jandl, A. Pöthig, M. Roverso, S. Bogialli, G. Tabacchi, E. Fois, E. Callone, and C. Maccato. Dalton Trans., 2021, 50(30), 10374-10385. https://doi.org/10.1039/D1DT01650D
J. R. Babcock, D. D. Benson, A. Wang, N. L. Edleman, J. A. Belot, M. V. Metz, and T. J. Marks. Chem. Vap. Deposition, 2000, 6(4), 180-183. https://doi.org/10.1002/1521-3862(200008)6:4<180::AID-CVDE180>3.0.CO;2-5
K. V. Zherikova, E. S. Vikulova, A. M. Makarenko, E. A. Rikhter, and L. N. Zelenina. Thermochim. Acta, 2020, 689, 178643. https://doi.org/10.1016/j.tca.2020.178643
E. S. Vikulova, D. A. Piryazev, K. V. Zherikova, N. I. Alferova, N. B. Morozova, and I. K. Igumenov. J. Struct. Chem., 2013, 54(5), 883-889. https://doi.org/10.1134/S0022476613050077
S. Brahma, M. Srinidhi, S. A. Shivashankar, T. Narasimhamurthy, and R. S. Rathore. J. Mol. Struct., 2013, 1035, 416-420. https://doi.org/10.1016/j.molstruc.2012.11.038
T. Hatanpää, J. Kansikas, I. Mutikainen, and M. Leskelä. Inorg. Chem., 2001, 40(4), 788-794. https://doi.org/10.1021/ic010160r
S. Delgado, A. Munoz, M. E. Medina, and C. J. Pastor. Inorg. Chim. Acta, 2006, 359(1), 109-117. https://doi.org/10.1016/j.ica.2005.10.018
C. Maccato, L. Bigiani, G. Carraro, A. Gasparotto, R. Seraglia, J. Kim, A. Devi, G. Tabacchi, E. Fois, G. Pace, V. Di Noto, and D. Barreca. Chem. – Eur. J., 2017, 23(71), 17954-17963. https://doi.org/10.1002/chem.201703423
Funding
The work was funded by the Russian Science Foundation (project 21-73-00252).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 8, 97037.https://doi.org/10.26902/JSC_id97037
Rights and permissions
About this article
Cite this article
Vikulova, E.S., Sukhikh, A.S., Mikhaylova, M.A. et al. STRUCTURE AND THERMAL PROPERTIES OF VOLATILE MIXED-LIGAND MAGNESIUM COMPLEXES: EFFECT OF TERT-BUTYL AND PHENYL SUBSTITUTES IN A FLUORINATED β-DIKETONATE. J Struct Chem 63, 1323–1332 (2022). https://doi.org/10.1134/S0022476622080133
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622080133