Abstract
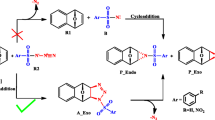
Arylsulfochlorination of β-aminopropioamidoximes is theoretically studied using estimations of thermodynamic parameters of the corresponding reactions, quantum chemical HOMO–LUMO analysis of molecular structures of reaction products. Full geometry optimization of the molecules is performed and vibrational frequencies are calculated with the Gaussian09 program using the DFT method at the B3LYP/6-31++G(d,p) level of theory. It is established that para-toluenesulfonates and para-nitrobenzenesulfonates of spiropyrazolinium compounds are thermodynamically most preferable in the arylsulfochlorination reactions of four studied β-aminopropioamidoximes in the presence of DIPEA, while the O-substitution products are most preferable in the reaction of β-(benzimidazol-1-yl)propioamidoxime arylsulfochlorination. The calculated results agree with experimental data and can be used to predict the structure and properties of amidoxime arylsulfochlorination products.





Similar content being viewed by others

REFERENCES
L. Kayukova, A. Vologzhanina, K. Praliyev, G. Dyusembaeva, G. Baitursynova, A. Uzakova, V. Bismilda, L. Chingissova, and K. Akatan. Molecules, 2021, 26(4), 967. https://doi.org/10.3390/molecules26040967
R. M. Srivastava, M. C. Pereira, W. W. M. Faustino, K. Coutinho, J. V. dos Anjos, and S. J. de Melo. Monatsh. Chem., 2009, 140(11), 1319-1324. https://doi.org/10.1007/s00706-009-0186-7
I. Doulou, C. Kontogiorgis, A. E. Koumbis, E. Evgenidou, D. Hadjipavlou-Litina, and K. C. Fylaktakidou. Eur. J. Med. Chem., 2014, 80, 145-153. https://doi.org/10.1016/j.ejmech.2014.04.040
A. Papastergiou, S. Perontsis, P. Gritzapis, A. E. Koumbis, M. Koffa, G. Psomas, and K. C. Fylaktakidou. Photochem. Photobiol. Sci., 2016, 15(3), 351-360. https://doi.org/10.1039/x0xx00000x
L. A. Kayukova, K. D. Praliyev, A. B. Myrzabek, and Z. N. Kainarbayeva. Russ. Chem. Bull., 2020, 69(3), 496-503. https://doi.org/10.1007/s11172-020-2789-4
L. A. Kayukova, G. P. Baitursynova, E. M. Yergaliyeva, B. A. Zhaksylyk, and N. S. Yelibayeva. Chem. J. Kaz., 2021, 2, 22-33. https://doi.org/10.51580/2021-1/2710-1185.25
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian09, Revision D.01. Wallingford, CT: Gaussian Inc., 2009.
A. D. Becke. J. Chem. Phys., 1993, 98(492), 5648-5652. https://doi.org/10.1063/1.464913
F. Odame and E. C. Hosten. Acta Chim. Slov., 2018, 65(3), 531-538. https://doi.org/10.17344/acsi.2017.4084
S. Masroor, M. Mobin, M. J. Alam, and S. Ahmad. RSC Adv., 2017, 7(37), 23182-23196. https://doi.org/10.1039/c6ra28426d
I. Franzoni, H. Yoon, J. A. García-López, A. I. Poblador-Bahamonde, and M. Lautens. Chem. Sci., 2018, 9(6), 1496-1509. https://doi.org/10.1039/C7SC04709F
E. Opoku, R. Tia, and E. Adei. J. Phys. Org. Chem., 2019, 32(10), e3992. https://doi.org/10.1002/poc.3992
L. Kayukova, K. Imanbekov, and K. Praliyev. Chem. J. Kaz., 2014, 2, 208-212.
A. Vörös, Z. Mucsi, Z. Baán, G. Timári, I. Hermecz, P. Mizsey, and Z. Finta. Org. Biomol. Chem., 2014, 12(40), 8036-8047. https://doi.org/10.1039/c4ob00854e.
X. Zhang, Y. Li, P. Guo, J. B. Le, Z. Y. Zhou, J. Cheng, and S. G. Sun. J. Catal., 2019, 376, 17-24. https://doi.org/10.1016/j.jcat.2019.06.037
S. Sevvanthi, S. Muthu, S. Aayisha, P. Ramesh, and M. Raja. Chem. Data Collect., 2020, 30, 100574. https://doi.org/10.1016/j.cdc.2020.100574
F. A. Bulat, J. S. Murray, and P. Politzer. Comput. Theor. Chem., 2021, 1199, 113192. https://doi.org/10.1016/j.comptc.2021.113192
M. Rocha, A. Di Santo, J. M. Arias, D. M. Gil, and A. B. Altabef. Spectrochim. Acta, Part A, 2015, 136, 635-643. https://doi.org/ 10.1016/j.saa.2014.09.077
A. Kumer, N. Sarker, S. Paul, and A. Zannat. Adv. J. Chem., Sect. A, 2019, 2(3), 190-202. https://doi.org/10.33945/SAMI/AJCA.2019.2.190202
G. A. Zhurko and D. A. Zhurko. ChemCraft, version 1.6. 2009. http://www.chemcraftprog.com (accessed Apr 18, 2021)
Funding
This work was funded by the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan, grant “Study of regioselectivity of arylsulfochlorination of β-aminopropioamidoximes reaction; in vitro antidiabetic and anti-tuberculosis product screening” (IRN AP08856440).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 12, pp. 2091-2096.https://doi.org/10.26902/JSC_id85842
Supplementary material
Rights and permissions
About this article
Cite this article
Yergaliyeva, E.M., Kayukova, L.A., Bazhykova, K.B. et al. COMPUTATIONAL STUDIES OF THE PRODUCTS OF TOSYLATION AND para-NITROBENZENESULFOCHLORINATION OF β-AMINOPROPIOAMIDOXIMES. J Struct Chem 62, 1969–1975 (2021). https://doi.org/10.1134/S0022476621120167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621120167