Abstract
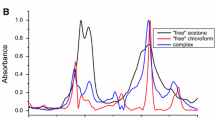
FTIR spectra of neat acetone (ACT), xylene isomers (o-xylene (OXY), m-xylene (MXY), and p-xylene (PXY)) and their binary solutions at various ACT molar concentrations are recorded in the range of 4000-400 cm–1. Although neat ACT is a mixture of monomers, dimers, and trimers, the number of dimers is larger than that of monomers/trimers, as suggested by a relatively higher intensity of the dimmer ν(C=O) band with respect to that of the monomers/trimers. The shifts suffered by some of the fundamental absorption bands of xylene isomers/ACT confirm the presence of (ACT)C=O⋯H(OXY/MXY/PXY aromatic C–H or methyl) and (ACT methyl)H⋯π(OXY/MXY/PXY) H-bonds in all the solutions but with different strengths, as suggested by different magnitudes of the vibrational band shifts. The asymmetric and symmetric stretching bands of either C–H or CH3 of OXY/PXY experience unequal force constants in ACTOXY and ACTPXY binary solutions with a 0.2 mole fraction of ACT. The νas(C–H) doublet due to the intramolecular coupling appears only in ACTOXY and ACTPXY binary solutions.





Similar content being viewed by others
REFERENCES
G. M. Desiraju. The Weak Hydrogen Bond in Structural Chemistry and Biology. New York: Oxford University Press, 1999.
G. F. Fabiola, S. Krishnaswamy, V. Nagarajan, and V. Pattabhi. Acta Crystallogr., Sect. D, 1997, 53, 316. https://doi.org/10.1107/S0907444997000383
W. Schindler, P. T. Sharko, and J. J. Jonas. Chem. Phys., 1982, 76, 3493. https://doi.org/10.1063/1.443449
B. Ancian, B. Tiffon, and J. E. Dubois. Chem. Phys., 1983, 74, 171. https://doi.org/10.1016/0301-0104(83)80020-2
E. Kneozinger and R. Wittenbeck. J. Mol. Spectrosc., 1984, 105, 314. https://doi.org/10.1016/0022-2852(84)90221-2
T. Shikata and N. Yoshida. J. Phys. Chem. A, 2012, 116, 4735. https://doi.org/10.1021/jp301520f
V. P. Bulychev, E. A. Svishcheva, and K. G. Tokhadze. Spectrochim. Acta, Part A, 2014, 117, 679. https://doi.org/10.1016/j.saa.2013.09.033
A. V. Afonin and M. A. Andriyankov. Zh. Org. Khim., 1988, 24, 1034. [In Russian]
H. Satonaka, K. Abe, and M. Hirota. Bull. Chem. Soc. Jpn., 1987, 60, 953. https://doi.org/10.1246/bcsj.60.953
H. Satonaka, K. Abe, and M. Hirota. Bull. Chem. Soc. Jpn., 1988, 61, 2031. https://doi.org/10.1246/bcsj.61.2031
I. E. Boldeskul, I. F. Tsymbal, E. V. Ryltsev, Z. Latajka, and A. J. Barnes. J. Mol. Struct., 1997, 436, 167. https://doi.org/10.1016/S0022-2860(97)00137-3
P. Hobza, V. Spirka, L. H. Selzle, and W. E. Schlag. J. Phys. Chem. A, 1998, 102, 25. https://doi.org/10.1021/jp973374w
J. W. Emsley, J. Feeney, and L. H. Sutcliffe. Progress in NMR spectroscopy. Oxford: Pergamon Press, 1978.
M. R. Jalilian. Spectrochim. Acta, Part A, 2008, 69, 812. https://doi.org/10.1016/j.saa.2007.05.032
L. I. De Beuckeleer and W. A. Herrebout. J. Phys. Chem. A, 2016, 120, 884. https://doi.org/10.1021/acs.jpca.5b10405
F. Kollipost, A. V. Domanskaya, and M. A. Suhm. J. Phys. Chem. A, 2015, 119, 2225. https://doi.org/10.1021/jp503999b
G. Arivazhagan, A. Elangovan, R. Shanmugam, R. Vijayalakshmi, and P. P. Kannan. Chem. Phys. Lett., 2015, 627, 101. https://doi.org/10.1016/j.cplett.2015.03.051
D. L. Jadhav, N. K. Karthick, P. P. Kannan, R. Shanmugam, A. Elangovan, and G. Arivazhagan, J. Mol. Struct., 2017, 1130, 497. https://doi.org/10.1016/j.molstruc.2016.10.055
S. K. Srivastava, A. K. Ojha, J. Koster, M. K. Shukla, J. Leszczynski, B. P. Asthana, and W. Kiefer. J. Mol. Struct., 2003, 661, 11. https://doi.org/10.1016/j.molstruc.2003.07.004
M. Musso, M. G. Giorgini, and H. Torii. J. Mol. Liq., 2009, 147, 37. https://doi.org/10.1016/j.molliq.2008.08.006
W. Schindler, P. T. Sharko, and J. J. Jonas. Chem. Phys., 1982, 76, 3493. https://doi.org/10.1063/1.443449
B. Ancian, B. Tiffon, and J. E. Dubois. Chem. Phys., 1983, 74, 171. https://doi.org/10.1016/0301-0104(83)80020-2
Y. Matsuda, K. Ohta, N. Mikami, and A. Fujii. Chem. Phys. Lett., 2009, 471, 50. https://doi.org/10.1016/j.cplett.2009.02.026
J. Guan, Y. Hu, M. Xie, and E. R. Bernstein. Chem. Phys. Lett., 2012, 405, 117. https://doi.org/10.1016/j.chemphys.2012.06.017
V. Arjunan, P. S. Balamourougane, I. Saravanan, and S. Mohan. Spectrochim. Acta, Part A, 2009, 74, 798. https://doi.org/10.1016/j.saa.2009.08.020
E. L. Hommel and H. C. Allen. Analyst, 2003, 128, 750. https://doi.org/10.1039/B301032P
R. Lindenmaier, N. K. Scharko, R. G. Tonkyn, K. T. Nguyen, S. D. Williams, and T. J. Johnson. J. Mol. Struct., 2017, 1149, 332. https://doi.org/10.1016/j.molstruc.2017.07.053
OriginPro 9.0. Northampton, MA, USA: OriginLab Corporation, n.d.
M. Wojdyr. J. Appl. Crystallogr., 2010, 43, 1126. https://doi.org/10.1107/S0021889810030499
J. D. Rogers, B. Rub, S. Goldman, and W. B. Person. J. Phys. Chem., 1981, 85, 3727. https://doi.org/10.1021/j150624a040
S. W. Han and K. Kim. J. Phys. Chem., 1996, 100, 17124. https://doi.org/10.1021/jp961538n
G. Varsanyi. Vibrational Spectra of Benzene Derivatives. New York: Academic Press, 1969. https://doi.org/10.1016/B978-0-12-714950-9.50007-7
P. Larkin. In: Infrared and Raman Spectroscopy, Principles and Spectral Interpretation. New York: Elsevier, 2011, 7-25. https://doi.org/10.1016/B978-0-12-386984-5.10002-3
J. H. S. Green. Spectrochim. Acta, Part A, 1970, 26, 1523. https://doi.org/10.1016/0584-8539(70)80213-6
T. Sangeetha, P. P. Kannan, N. K. Karthick, A. Mahendraprabu, and G. Arivazhagan. J. Mol. Liq., 2020, 312, 113406. https://doi.org/10.1016/j.molliq.2020.113406
D. R. Lide. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press, 2005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 12, pp. 2028-2038.https://doi.org/10.26902/JSC_id84483
Supplementary material
Rights and permissions
About this article
Cite this article
Naganandhini, S.P., Sangeetha, T. & Arivazhagan, G. FTIR SPECTRAL STUDIES OF THE BINARY SOLUTIONS OF ACETONE WITH XYLENE ISOMERS. J Struct Chem 62, 1907–1917 (2021). https://doi.org/10.1134/S0022476621120106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621120106