Abstract
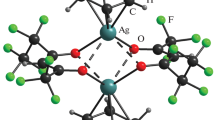
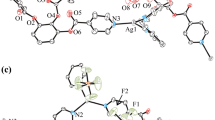
Silver β-diketonates [Ag(RC(O)CHC(O)R′)]∞ with bulky terminal substituents are synthesized and characterized: R = C(OCH3)(CH3)2, R′ = CF3 (1), R = R′ = C(CH3)3 (2). Complex 1 is obtained for the first time. Structures of compounds are determined by single crystal X-ray diffraction. For complex 1 the crystallographic data are: space group \(P\bar{1}\), a = 5.8012(6) Å, b = 9.1717(10) Å, c = 9.9251(11) Å, α = 73.722(4)°, β = 78.377(4)°, γ = 87.336(4)°, V = 496.49(9) Å3, Z = 2, dcalc = 2.134 g/cm3, μ = 2.059 mm–1; for complex 2: space group C2, a = 22.1700(6) Å, b = 5.84530(10) Å, c = 9.3249(2) Å, β = 104.9670(10)°, V = 1167.42(5) Å3, Z = 4, dcalc = 1.656 g/cm3, μ = 1.701 mm–1. Both compounds are chain coordination polymers. Anions are coordinated in a bidentate-cyclic fashion. One of carbonyl oxygen atoms and the carbon atom in the CH-group perform the bridging function. In complex 2, the coordination environment of silver is a distorted vacant trigonal bipyramid (CN = 4). In complex 1, it is a tetragonal pyramid due to the additional coordination of the methoxy oxygen atom of the neighboring anion (CN = 5). The structure and thermal stability of the synthesized complexes are compared with those of the closest analogue (R = C(CH3)3, R′ = CF3), which allowed us to reveal the effect of terminal substituents in the β-diketonate ligand.





Similar content being viewed by others
REFERENCES
H. X. Ruan. 1,1,1-(trifluoroacetylacetonato)silver(I) Used for Photochemical and Thermal Deposition of Silver and Silver Oxide Film and Its Kinetics: M. Sc. (Chem.) Dissertation. Bumaby, BC, Canada: Simon Fraser University, 2007.
J. D. Warner, M. Pevzner, C. J. Dean, D. E. Kranbuehl, J. L. Scott, S. T. Broadwater, D. W. Thompson, and R. E. Southward. J. Mater. Chem., 2003, 13, 1847-1852. https://doi.org/10.1039/B212546C
G. Carotenuto, M. Palomba, A. Longo, S. De Nicola, and L. Nicolais. Sci. Eng. Compos. Mater., 2011, 18, 187-190. https://doi.org/10.1515/secm.2011.030
G. Calabrese, S. Petralia, D. Franco, G. Nocito, C. Fabbi, L. Forte, S. Guglielmino, S. Squarzoni, F. Traina, and S. Conoci. Mater. Sci. Eng. C, 2021, 118, 111394. https://doi.org/10.1016/j.msec.2020.111394
A. O. Rybaltovskii, V. G. Arakcheev, A. N. Bekin, A. F. Danilyuk, V. I. Gerasimova, N. V. Minaev, E. N. Golubeva, O. O. Parenago, and V. N. Bagratashvili. Russ. J. Phys. Chem. B, 2015, 9, 1137-1142. https://doi.org/10.1134/S1990793115080096
S. E. Bozbağ and C. Erkey. J. Supercrit. Fluids, 2015, 96, 298-312. https://doi.org/10.1016/j.supflu.2014.09.036
C. N. Chen, T. Y. Dong, T. C. Chang, M. C. Chen, H. L. Tsai, and W. S. Hwang. J. Mater. Chem. C, 2013, 1, 5161-5168. https://doi.org/10.1039/C3TC30911H
A. Ievtushenko, V. Karpyna, J. Eriksson, I. Tsiaoussis, I. Shtepliuk, G. Lashkarev, R.Yakimova, and V. Khranovskyy. Superlattices Microstruct., 2018, 117, 121-131. https://doi.org/10.1016/j.spmi.2018.03.029
P. Piszczek and A. Radtke. Silver Nanoparticles Fabricated Using Chemical Vapor Deposition and Atomic Layer Deposition Techniques: Properties, Applications And Perspectives: Review. In: Noble and Precious Metals - Properties, Nanoscale Effects and Applications / Eds. M. S. Seehra and A. D Bristow. London: IntechOpen, 2018, 187-213. https://doi.org/10.5772/intechopen.71571
O. Aschenbrenner, S. Kemper, N. Dahmen, K. Schaber, and E. Dinjus. J. Supercrit. Fluids, 2007, 41, 179-186. https://doi.org/10.1016/j.supflu.2006.10.011
S. Yoda, Y. Mizuno, T. Furuya, Y. Takebayashi, K. Otake, T. Tsuji, and T. Hiaki. J. Supercrit. Fluids, 2008, 44, 139-147. https://doi.org/10.1016/j.supflu.2007.11.002
A. J. Blake, N. R. Champness, S. M. Howdle, K. S. Morley, P. B. Webb, and C. Wilson. CrystEngComm, 2002, 4, 88-92. https://doi.org/10.1039/B200491G
K. Akhbari and A. Morsali. Cryst. Growth Des., 2007, 7, 2024-2030. https://doi.org/10.1021/cg0704652
F. Marandi, M. Ghadermazi, A. Marandi, I. Pantenburg, and G. Meyer. J. Mol. Struct., 2011, 1006, 136-141. https://doi.org/10.1016/j.molstruc.2011.08.059
C. Xu, T. S. Corbitt, M. J. Hampden-Smith, T. T. Kodas, and E. N. Duesler. J. Chem. Soc., Dalton Trans., 1994, 2841-2849. https://doi.org/10.1039/DT9940002841
W. J. Evans, D. G. Giarikos, and D. Josell. Inorg. Chem., 2003, 42, 8255-8261. https://doi.org/10.1021/ic034649r
D. Henderson, F. White, and P. Tasker. CCDC 1410146: Experimental Crystal Structure Determination, 2015. https://doi.org/10.5517/cc1jbclt
S. Patterson, D. Henderson, and P. Tasker. CCDC 1410235: Experimental Crystal Structure Determination, 2015. https://doi.org/10.5517/cc1jbggs
I. S. Fedoseev, E. S. Vikulova, I. Y. Ilin, A. I. Smolentsev, M. R. Gallyamov, and N. B. Morozova. J. Struct. Chem., 2016, 57(8), 1667-1670. https://doi.org/10.1134/S0022476616080242
I. K. Igumenov, T. V. Basova, and V. R. Belosludov. Volatile Precursors for Films Deposition: Vapor Pressure, Structure and Thermodynamics. In: Application of Thermodynamics to Biological and Materials Science / Ed. M. Tadashi. London: IntechOpen, 2011, 521-546. https://doi.org/10.5772/13356
S. C. Ngo, K. K. Banger, P. J. Toscano, and J. T. Welch. Polyhedron, 2002, 21, 1289-1297. https://doi.org/10.1016/S0277-5387(02)00980-4
V. P. Fadeeva, V. D. Tikhova, O. N. Nikulicheva, I. I. Oleynik, and I. V. Oleynik. J. Struct. Chem., 2010, 51, 186-191. https://doi.org/10.1007/s10947-010-0211-z
V. P. Fadeeva, V. D. Tikhova, and O. N. Nikulicheva. J. Anal. Chem., 2008, 63, 1094. https://doi.org/10.1134/S1061934808110142
Powder Diffraction File, release 2010, International Centre for Diffraction Data, Pennsylvania, USA. http://www.icdd.com/products/pdf2.htm
T. Li, M. T. Gamer, M. Scheer, S. N. Konchenko, and P. W. Roesky. Chem. Comm., 2013, 49, 2183-2185 (Supplementary information). https://doi.org/10.1039/C3CC38841G
Bruker Apex3 software suite: Apex3, SADABS-2016/2 and SAINT, Version 2018.7-2. Madison, WI: Bruker AXS Inc., 2017.
G. M. Sheldrick. Acta Crystallogr., Sect. A., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3-8. https://doi.org/10.1107/S2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339-341. https://doi.org/10.1107/S0021889808042726
S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell, and D. Avnir. Coord. Chem. Rev., 2005, 249, 1693-1708. https://doi.org/10.1016/j.ccr.2005.03.031
M. Llunell, D. Casanova, J. Cirera, P. Alemany, and S. Alvarez. SHAPE, Version 2.1. Spain: Universitat de Barcelona, 2013. http://www.ee.ub.edu/
J. A. Darr, M. Poliakoff, A. J. Blake, and W. S. Li. Inorg. Chem., 1998, 37, 5491-5496. https://doi.org/10.1021/ic971206c
L. Zanotto, F. Benetollo, M. Natali, G. Rossetto, P. Zanella, S. Kaciulis, and A. Mezzi. Chem. Vapor Depos., 2004, 10, 207-213. https://doi.org/10.1002/cvde.200306290
F. Marandi, M. Ghadermazi, A. Marandi, I. Pantenburg, and G. Meyer. J. Mol. Struct., 2011, 1006, 136-141. https://doi.org/10.1016/j.molstruc.2011.08.059
H. Liu, S. Battiato, A. L. Pellegrino, P. Paoli, P. Rossi, C. Jiménez, G. Malandrino, and D. Muñoz-Rojas. Dalton Trans., 2017, 46, 10986-10995. https://doi.org/10.1039/C7DT01647F
J. L. Jin, Y. L. Shen, Y. P. Xie, and X. Lu. CrystEngComm, 2018, 20, 2036-2042. https://doi.org/10.1039/C8CE00243F
Funding
The work was supported by grant MK-6148.2021.1.3 of the President of the Russian Federation for young scientists–candidates of science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 12, pp. 1953-1962.https://doi.org/10.26902/JSC_id83846
Rights and permissions
About this article
Cite this article
Gulyaev, S.A., Vikulova, E.S., Sukhikh, T.S. et al. STRUCTURES AND THERMAL PROPERTIES OF SILVER(I) β-DIKETONATES WITH BULKY TERMINAL SUBSTITUENTS. J Struct Chem 62, 1836–1845 (2021). https://doi.org/10.1134/S0022476621120039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621120039