Abstract
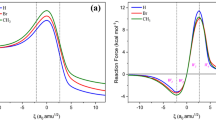
In the present article, a DFT approach is used at the M06-2X/6-311G(d,p) level of theory to survey [2+4] Diels–Alder cycloaddition reactions of anthracene with C2X2 (X = H, F, Cl, Me). To illustrate the interaction between two fragments in transition states and products, the energy decomposition analysis is employed. The stability of two feasible products are contrasted. The substituent impact barrier height (ΔE‡) and thermodynamic parameters (ΔG‡ and ΔH‡) of this reaction are found. To check the advancement of the reactions, Wiberg bond indices are applied. Furthermore, the synchronicity values of this reaction are identified.



Similar content being viewed by others
REFERENCES
F. Fringuelli and A. Taticchi. The Diels-Alder Reaction. Selected Practical Methods. John Wiley & Sons: New York, 2002. https://doi.org/10.1002/0470845813
I. Fleming. Pericyclic Reactions. Oxford University Press: New York, 1999.
K. C. Nicolau, S. A. Snyder,T. Montagnon, and G. Vassilikogiannakis. Angew. Chem., Int. Ed., 2002, 41, 1668. https://doi.org/10.1002/1521-3773(20020517)41:10%3C1668::AID-ANIE1668%3E3.0.CO;2-Z
O. Diels and K. Alder. Justus Liebigs Ann. Chem., 1928, 98, 460. https://doi.org/10.1002/jlac.19284600106
W. Carruthers. Some Modern Methods of Organic Synthesis, 2nd ed. Cambridge University Press: Cambridge, UK, 1978.
W. Carruthers. Cycloaddition Reactions in Organic Synthesis. Pergamon: Oxford, UK, 1990.
P. Geerlings, F. D. Proft, and W. Langenaeker. Chem. Rev., 2003, 103, 1793. https://doi.org/10.1021/cr990029p
D. H. Ess, G. O. Jones, and K. N. Houk. Adv. Synth. Catal., 2006, 348, 2337. https://doi.org/10.1002/adsc.200600431
C. M. Ormachea, P. M. E. Mancini, M. N. Kneeteman, and L. R. Domingo. Comput. Theor. Chem., 2015, 1072, 37. https://doi.org/10.1016/j.comptc.2015.08.024
A. M. Sarotti. Org. Biomol. Chem., 2014, 12, 187. https://doi.org/10.1039/C3OB41628C
P. M. E. Mancinia, M. N. Kneeteman, M. Cainelli, C. M. Ormachea, and L. R. Domingo. J. Mol. Struct., 2017, 1147, 155. https://doi.org/10.1016/j.molstruc.2017.06.109
R. Breslow, U. Maitra, and D. Rideout. Tetrahedron Lett., 1983, 24, 1901. https://doi.org/10.1016/S0040-4039(00)81801-8
C. Cativiela, J. I. Garcia, J. A. Mayoral, A. Avenoza, J. M. Peregrina, and M. A. Roy. J. Phys. Org. Chem., 1991, 4, 48. https://doi.org/10.1002/poc.610040108
C. Cativiela, J. I. García, J. A. Mayoral, and L. Salvatella. J. Chem. Soc., Perkin Trans., 1994, 847. https://doi.org/10.1039/P29940000847
M. F. Ruiz-López, X. Assfeld, J. I. X. García, J. A. Mayoral, and L. Salvatella. J. Am. Chem. Soc., 1993, 115, 8780. https://doi.org/10.1021/ja00072a035
H. J. Schneider and N. K. Sangwan. Angew. Chem., Int. Ed., 1987, 26, 896. https://doi.org/10.1002/anie.198708961
R. Bini, C. Chiappe, V. L. Mestre, C. S. Pomellic, and T. Welton. Org. Biomol. Chem., 2008, 6, 2522. https://doi.org/10.1039/b802194e
C. Chiappe, M. Malvaldi, and C. S. Pomelli. Green Chem., 2010, 12, 1330. https://doi.org/10.1039/c0gc00074d
R. Bini, C. Chiappe, V. L. Mestre, and C. S. Pomelli, T. Welton. Theor. Chem. Acc., 2009, 123, 347. https://doi.org/10.1007/s00214-009-0525-0
E. S. Ansari, R. Ghiasi, and A. Forghaniha. Struct. Chem., 2019, 30, 877–885. https://doi.org/10.1007/s11224-018-1241-y
E. S. Ansari, R. Ghiasi, and A. Forghaniha. Chem. Methodol., 2020, 4, 220. https://dx.doi.org/10.33945/SAMI/CHEMM/2020.3.1
G. H. Sarova and M. N. Berberan-Santos. Chem. Phys. Lett., 2004, 397, 402. https://doi.org/10.1016/j.cplett.2004.09.005
T. Tsuda, T. Ishida, T. Nogami, S. Kurono, and M. Ohashi. J. Chem. Soc., Chem. Commun., 1993, 1296. https://doi.org/10.1039/C39930001296
Y. Rubin, S. Khan, D. I. Freedberg, and C. Yeretzian. J. Am. Chem. Soc., 1993, 115, 344. https://doi.org/10.1021/ja00054a049
M. Randic. Chem. Rev., 2003, 103, 3449. https://doi.org/10.1021/cr9903656
M. E. Hayes, H. Shinokubo, and R. L. Danheiser. Org. Lett., 2005, 7, 3917. https://doi.org/10.1021/ol051372l
B. Liu, Q. Hu, F. Yang, X. Zheng, and Y. Hu. Chin. Chem. Lett., 2020, 31(5), 1305. https://doi.org/10.1016/j.cclet.2019.10.003
M. A. Sultan, U. Karama, A. I. Almansour, and S. M. Soliman. Molecules, 2016, 21, 1277. https://doi.org/10.3390/molecules21101277
S. Agopcan, N. Çelebi-Ölçüm, M. N. Üçışık, A. Sanyala, and V. Aviyente. Org. Biomol. Chem., 2011, 9, 8079. https://doi.org/10.1039/c1ob06285a
W. C. Herndon. J. Chem. Soc., Chem. Commun., 1977, 817. https://doi.org/10.1039/c39770000817
W. C. Herndon, and M. L. Ellzey Jr. J. Am. Chem. Soc., 1974, 96, 6631. https://doi.org/10.1021/ja00828a015
A. F. Khasanov, D. S. Kopchuk, I. S. Kovalev, O. S. Taniya, K.Giri, P. A. Slepukhin, S. Santra, M. Rahman, A. Majee, V. N. Charushin, and O. N. Chupakhin. New J. Chem., 2017, 41, 2309. https://doi.org/10.1039/C6NJ02956F
Q. C. Zhang, J. Lv, S. Li, and S. Luo. Org. Lett., 2018, 20, 2269. https://doi.org/10.1021/acs.orglett.8b00619
J. P. Hernández-Mancera, F. Núñez-Zarur, and R. Vivas-Reyes. ChemistryOpen, 2020, 9, 748. https://doi.org/10.1002/open.202000137
A. Stockmann, J. Kurzawa, N. Fritz, N. Acar, S. Schneider, J. Daub, R. Engl, and T. Clark. J. Phys. Chem. A, 2002, 106, 7958. https://doi.org/10.1021/jp0142987
M. Ottonelli, M. Piccardo, D. Duce, S. Thea, and G. Dellepiane. J. Phys. Chem. A, 2012, 116, 611. https://doi.org/10.1021/jp2084764
Y.-H. Cheng, Y. Fang, X. Zhao, L. Liu, and Q.-X. Guo. Bull. Chem. Soc. Jpn., 2002, 75, 1715. https://doi.org/10.1246/bcsj.75.1715
F. Pichierri. Theor. Chem. Acc., 2017, 136, 114-123. https://doi.org/10.1007/s00214-017-2144-5
G. S. Remya and C. H. Suresh. Phys. Chem. Chem. Phys., 2016, 18, 20615. https://doi.org/10.1039/C6CP02936A
H. Szatylowicz, A. Jezuita, T.Siodła, K. S. Varaksin, M. A. Domanski, K. Ejsmont, and T. M. Krygowski. ACS Omega, 2017, 2, 7163. https://doi.org/10.1021/acsomega.7b01043
R. Ghiasi and A. Zamani. J. Chin. Chem. Soc., 2017, 64, 1340. https://doi.org/10.1002/jccs.201700172
R. Ghiasi, H. Pasdar, and S. Fereidoni. Russ. J. Inorg. Chem., 2016, 61, 327. https://doi.org/10.1134/S0036023616030104
R. Ghiasi and A. Heydarbeighi. Russ. J. Inorg. Chem., 2016, 61, 985. https://doi.org/10.1134/S0036023616080088
R. Ghiasi, H. Pasdar, and F. Irajizadeh. J. Chil. Chem. Soc., 2015, 60, 2740. https://doi.org/10.4067/S0717-97072015000400020
R. Ghiasi, S. Abdolmohammadi, and S. Moslemizadeh. J. Chin. Chem. Soc., 2015, 62, 898. https://doi.org/10.1002/jccs.201500249
A. Peikari, R. Ghiasi, and H. Pasdar. Russ. J. Phys. Chem. A, 2015, 89, 250. https://doi.org/10.1134/S0036024415020260
M. Z. Fashami, and R. Ghiasi. J. Struct. Chem., 2015, 56, 1474. https://doi.org/10.1134/S0022476615080041
R. Ghiasi and A. Boshak. J. Mex. Chem. Soc., 2013, 57, 8. https://doi.org/10.29356/jmcs.v57i1.229
H. Pasdar and R. Ghiasi. Main Group Chem., 2009, 8, 143. https://doi.org/10.1080/10241220902977653
A. N. Egorochkin, O. V. Kuznetsova, N. M. Khamaletdinova, and L. G. Domratcheva-Lvova. Inorg. Chim. Acta, 2018, 471, 148. https://doi.org/10.1016/j.ica.2017.10.021
H. Anane, S.E. Houssame, A. E. Guerraze, A. Guermoune, A. Boutalib, A. Jarid, I. Nebot-Gil, and F. Tomás. Cent. Eur. J. Chem., 2008, 6, 400. https://doi.org/10.2478/s11532-008-0029-0
D. M. Denning and D. E. Falvey. J. Org. Chem., 2017, 82, 1552. https://doi.org/10.1021/acs.joc.6b02755
M. Wesolowski and T. Konarski. J. Therm. Anal. Calorim., 1999, 55, 995. https://doi.org/10.1023/A:1010162607157
F. Jia, L.-P. Yang, D.-H. Li, and W. Jiang. J. Org. Chem., 2017, 82, 10444. https://doi.org/10.1021/acs.joc.7b01914
T. H. Lowry and K. S. Richardson. Mechanism and Theory in Organic Chemistry, 3d ed. Harper Collins: New York, 1987.
S. Berson, S. Cecioni, M. Billon, Y. Kervella, R. Bettignies, S. Bailly, and S. Guillerez. Sol. Energy Mater. Sol. Cells, 2010, 94, 699. https://doi.org/10.1016/j.solmat.2009.12.028
M. A. Solomos, T. A. Watts, and J. A. Swift. Cryst. Growth Des., 2017, 17, 5065. https://doi.org/10.1021/acs.cgd.7b00757
J. Ohshita, K. Hiroyuki, A. Takata, I. Toshiyuki, A. Kunai, N. Nhta, K. Komaguchi, M. Shiotani, A. Adachi,K. Sakamaki, and K. Okita. Organometallics, 2001, 20, 4800. https://doi.org/10.1021/om0103254
S. Abou-Hatab, V.A. Spata, and S. Matsika. J. Phys. Chem. A, 2017, 121, 1213. https://doi.org/10.1021/acs.jpca.6b12031
J. Jung, J. Jo, and A. Dinescu. Org. Process Res. Dev., 2017, 21, 1689. https://doi.org/10.1021/acs.oprd.7b00269
C.-T. Cao, L.-Y. Wang, and C.-Z. Cao. Chin. J. Chem. Phys., 2018, 31, 45. https://doi.org/10.1063/1674-0068/31/cjcp1704077
C. R. Caiser, E. A. Basso, and R. Rittner. Magn. Reson. Chem., 2001, 39, 643. https://doi.org/10.1002/mrc.875
K. A. Manbeck, N. C. Boaz, N. C. Bair, A. M. S. Sanders, and A. L. Marsh. J. Chem. Educ., 2011, 88, 1444. https://doi.org/10.1021/ed1010932
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalman, V. Barone,B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi,M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma,V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman,J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian09, Revision A.02. Gaussian: Wallingford CT, 2009.
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople. J. Chem. Phys., 1980, 72, 650. https://doi.org/10.1063/1.438955
A. D. McLean and G. S. Chandler. J. Chem. Phys., 1980, 72, 5639. https://doi.org/10.1063/1.438980
Y. Zhao, and D. G. Truhla. J. Phys. Chem. A, 2006, 110, 5121. https://doi.org/10.1021/jp060231d
A. E. Reed, L. A. Curtiss, and F. Weinhold. Chem. Rev., 1988, 88, 899. https://doi.org/10.1021/cr00088a005
E. D. Glendening, A. E. Reed, J. E. Carpenter, and F. Weinhold. NBO Version 3.1. Madison, 1988.
K. Morokuma. J. Chem. Phys., 1971, 55, 1236. https://doi.org/10.1063/1.1675562
T. Ziegler and A. Rauk. Theor. Chim. Acta, 1977, 46, 1. https://doi.org/10.1007/BF02401406
F. M. Bickelhaupt, and E. J. Baerends. Kohn-Sham Density Functional Theory: Predicting and Understanding Chemistry. In: Reviews in Computational Chemistry / Eds. K. B. Lipkowitz and D. B. Boyd. John Wiley & Sons: Weinheim, 2000, Vol. 15.
T. Lu and F. Chen. J. Comput. Chem., 2012, 33, 580. https://doi.org/10.1002/jcc.22885
T. Lu and F. Chen. J. Mol. Graphics Modell., 2012, 38, 314. https://doi.org/10.1016/j.jmgm.2012.07.004
C. R. Zhang, H. S. Chen, and G. H. Wang. Chem. Res. Chin. Univ., 2004, 20, 640.
H. Cheng, J. Feng, A. Ren, and J. Liu. Acta Chim. Sin., 2002, 60, 830.
Y. Sun, X. Chen, L. Sun, X. Guo, and W. Lu. Chem. Phys. Lett., 2003, 381, 397. https://doi.org/10.1016/j.cplett.2003.09.115
P. K. Chattaraj and S. Sengupta. J. Phys. Chem., 1996, 100, 16126. https://doi.org/10.1021/jp961096f
T. K. Ghanty and S. K. Ghosh. J. Phys. Chem., 1996, 100, 12295. https://doi.org/10.1021/jp960276m
P. K. Chattaraj, P. Fuentealba, B. Gomez, and R. Contreras. J. Am. Chem. Soc., 2000, 122, 348. https://doi.org/10.1021/ja992337a
P. K. Chattaraj, D. R. Roy, and S. Giri. Comput. Lett., 2007, 3, 223. https://doi.org/10.1163/157404007782913336
R. G. Pearson. Chemical Hardness. Wiley-VCH: Oxford, 1997. https://doi.org/10.1002/3527606173
R. G. Parr and W. Yang. Density-Functional Theory of Atoms and Molecules. Oxford University Press: New York, 1989.
R. G. Parr, L. v. Szentpály, and S. Liu. J. Am. Chem. Soc., 1999, 121, 1922. https://doi.org/10.1021/ja983494x
P. W. Ayers and R. G. Parr. J. Am. Chem. Soc., 2000, 422, 2010. https://doi.org/10.1021/ja9924039
R. G. Parr and P. K. Chattaraj. J. Am. Chem. Soc., 1991, 113, 1854. https://doi.org/10.1021/ja00005a072
R. G. Pearson. J. Chem. Educ., 1987, 64, 561. https://doi.org/10.1021/ed064p561
R. G. Pearson. Acc. Chem. Res., 1993, 26, 250. https://doi.org/10.1021/ar00029a004
R. G. Pearson. J. Chem. Educ., 1999, 76, 267. https://doi.org/10.1021/ed076p267
E. Chamorro, P. K. Chattaraj, and P. Fuentealba. J. Phys. Chem. A, 2003, 107, 7068. https://doi.org/10.1021/jp035435y
R. Parthasarathi, M. Elango, V. Subramanian, and P. K. Chattaraj. Theor. Chem. Acc., 2005, 113, 257. https://doi.org/10.1007/s00214-005-0634-3
J. Padmanabhan, R. Parthasarathi, V. Subramanian, and P. K. Chattaraj. J. Phys. Chem. A, 2007, 111, 1358. https://doi.org/10.1021/jp0649549
R. G. Pearson. J. Org. Chem., 1989, 54, 1430. https://doi.org/10.1021/jo00267a034
R. G. Parr and R. G. Pearson. J. Am. Chem. Soc., 1983, 105, 7512. https://doi.org/10.1021/ja00364a005
K. B. Wiberg. Tetrahedron, 1968, 24, 1083. https://doi.org/10.1016/0040-4020(68)88057-3
A. Moyano, M. A. Pericas, and E. Valenti. J. Org. Chem., 199, 54, 573. https://doi.org/10.1021/jo00264a014
M. Manoharan and P. Venuvanalingam. J. Mol. Struct.: THEOCHEM, 1997, 394, 41. https://doi.org/10.1016/S0166-1280(96)04899-3
M. Manoharan and P. Venuvanalingam. J. Chem. Soc., Perkin Trans., 1997, 1799. https://doi.org/10.1039/a700250e
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 10, pp. 1656-1666.https://doi.org/10.26902/JSC_id80799
Supplementary material
Rights and permissions
About this article
Cite this article
Ahraminejad, M., Ghiasi, R., Mohtat, B. et al. SUBSTITUENT EFFECT IN [2+4] DIELS–ALDER CYCLOADDITION REACTIONS OF ANTHRACENE WITH C2X2 (X = H, F, Cl, Me): A COMPUTATIONAL INVESTIGATION. J Struct Chem 62, 1551–1562 (2021). https://doi.org/10.1134/S0022476621100097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621100097