Abstract
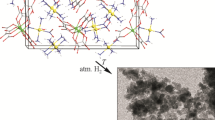
New complex salts [Pd(NH3)4][Pd(NH3)3NO2][RhOx3]·H2O and Na3[RhOx3]·4H2O are prepared. The crystal structures of obtained compounds are determined by XRD. Complex salt [Pd(NH3)4] [Pd(NH3)3NO2][RhOx3]·H2O is studied by powder XRD, IR spectroscopy, and elemental analysis. The state of rhodium(III) in oxalate solutions is studied by NMR. Thermal behavior of [Pd(NH3)4][Pd(NH3)3NO2] [RhOx3]·H2O in various gas atmospheres shows that final thermolysis products in reducing and inert atmospheres are solid solutions Pd1–xRhx.








Similar content being viewed by others
REFERENCES
C. Te Hsieh, Y. F. Chen, and P. Y. Yu. Int. J. Hydrogen Energy, 2015, 40, 14857-14865, DOI: 10.1016/j.ijhydene.2015.09.035.
F. Mauriello, H. Ariga-Miwa, E. Paone, R. Pietropaolo, S. Takakusagi, and K. Asakura. Catal. Today, 2019, 1-7, DOI: 10.1016/j.cattod.2019.06.071.
P. A. J. Bagot, K. Kruska, D. Haley, X. Carrier, E. Marceau, M. P. Moody, and G. D. W. Smith. J. Phys. Chem., 2014, 118, 26130-26138, DOI: 10.1021/jp508144z.
H. Fang, J. Yang, M. Wen, and Q. Wu. Adv. Mater., 2018, 30, 1-10, DOI: 10.1002/adma.201705698.
S. Shan, J. Luo, L. Yang, and C. J. Zhong. Catal. Sci. Technol., 2014, 4, 3570-3588, DOI: 10.1039/c4cy00469h.
W. Zhang, S. Shan, J. Luo, A. Fisher, J. F. Chen, C. J. Zhong, J. Zhu, and D. Cheng. J. Phys. Chem., 2017, 121, 11010-11020, DOI: 10.1021/acs.jpcc.6b10814.
Z. Wang and X. Bai. J. Mol. Struct., 2020, 1219, 128538, DOI: 10.1016/j.molstruc.2020.128538.
Z. Zhou, J. Ouyang, H. Yang, and A. Tang. Appl. Clay Sci., 2016, 121-122, 63-70, DOI: 10.1016/j.clay.2015.12.017.
F. Le Normand, J. Barrault, R. Breault, L. Hilaire, and A. Kiennemann. J. Phys. Chem., 1991, 95, 257-269, DOI: 10.1021/j100154a050.
A. A. Vedyagin, A. M. Volodin, V. O. Stoyanovskii, R. M. Kenzhin, E. M. Slavinskaya, I. V. Mishakov, P. E. Plyusnin, and Y. V. Shubin. Catal. Today, 2014, 238, 80-86, DOI: 10.1016/j.cattod.2014.02.056
H. Wei, X. Wei, X. Yang, G. Yin, A. Wang, X. Liu, Y. Huang, and T. Zhang. Chin. J. Catal., 2015, 36, 160-167, DOI: 10.1016/S1872-2067(14)60254-0.
P. E. Plyusnin, E. V. Makotchenko, Y. V. Shubin, I. A. Baidina, I. V. Korolkov, L. A. Sheludyakova, and S. V. Korenev. J. Mol. Struct., 2015, 1100, 174-179, DOI: 10.1016/j.molstruc.2015.07.023.
I. A. Garkul, A. V. Zadesenets, I. V. Korolkov, I. A. Baidina, and S. V. Korenev. J. Struct. Chem., 2020, 61(5), 719-726, DOI: 10.1134/S0022476620050078.
S. V. Korenev, A. B. Venediktov, Y. V. Shubin, S. A. Gromilov, and K. V. Yusenko. J. Struct. Chem., 2003, 44(1), 46-59, DOI: 10.1023/A:1024980930337.
F. Khosravi and H. Mansouri-Torshizi. J. Biomol. Struct. Dyn., 2018, 36, 512-531, DOI: 10.1080/07391102.2017.1281171.
A. Zadesenets, E. Filatov, P. Plyusnin, I. Baidina, V. Dalezky, Y. Shubin, S. Korenev, and A. Bogomyakov. Polyhedron, 2011, 30, 1305-1312, DOI: 10.1016/j.poly.2011.02.012.
A. N. Nana, D. Haynes, H. Vezin, C. Minaud, P. Shankhari, B. P. T. Fokwa, and J. Nenwa. J. Mol. Struct., 2020, 1220, 1-9, DOI: 10.1016/j.molstruc.2020.128642.
A. V. Zadesenets, I. A. Garkul, E. Y. Filatov, P. E. Plyusnin, T. N. Filippov, T. I. Asanova, I. V. Korolkov, I. A. Baidina, I. P. Asanov, and S. V. Korenev. J. Therm. Anal. Calorim., 2019, 138, 111-121, DOI: 10.1007/s10973-019-08254-0.
Sintez Kompleks. Soyed. Metal. Platin. gruppy: Spravochnik (Synth. Complex Compd. Platinum Group Met.: A Handbook) [in Russian] / Ed. I. I. Chernyaev. Nauka: Moscow, 1964.
Powder Diffraction File, PDF-2/Release 2009. International Centre for Diffraction Date: USA, 2009.
G. M. Sheldrick. SHELXTL-97, Progr. Refinement Cryst. Struct. Univ. of Göttingen: Göttingen, Germany, 1998.
NETZSCH Proteus Thermal Analysis, v. 6.1.0. Selb/ NETZSCH-Gerätebau GmbH: Bayern, Germany, 2013.
Bruker Apex3 software suite: Apex3, SADABS v. 2016/2, TWINABS and SAINT v. 2018.7-2. Bruker AXS: Madison, WI, 2017.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2015, 71, 3-8.
G. M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3-8.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339.
A. B. Venediktov, A. A. Kazakova, M. A. Fedotov, and A. B. Belyaev. Koord. Khim. 1989, 15, 822-824.
F. P. Boer, V. B. Carter. and J. W. Turley. Inorg. Chem., 1971, 10, 651-661.
R. Kuroda. Inorg. Chem., 1991, 30, 4954-4959, DOI: 10.1021/ic00026a020.
L. A. Kushch, L. S. Kurochkina, E. B. Yagubskii, G. V. Shilov, S. M. Aldoshin, V. A. Emelyanov, Y. N. Shvachko, V. S. Mironov, D. Schaniel, T. Woike, C. Carbonera, and C. Mathonière. Eur. J. Inorg. Chem., 2006, 4074-4085, DOI: 10.1002/ejic.20060027.
R. Tian, Y. Yan, C. Zhang, L. Wang, and Q. Pan. Acta Crystallogr., Sect. E: Crystallogr. Commun., 2012, 68, m914-m915, DOI: 10.1107/S1600536812026414.
K. Nakamoto. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Wiley-Interscience: New York, 1986.
Funding
The work was funded by the Russian Science Foundation (project No. 16-13-10192).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 5, pp. 839-851.https://doi.org/10.26902/JSC_id72911
Rights and permissions
About this article
Cite this article
Gladysheva, M.V., Plyusnin, P.E., Vorobyeva, S.N. et al. COMPLEX SALT [Pd(NH3)4][Pd(NH3)3NO2][RhOx3]·H2O AS A PROSPECTIVE PRECURSOR OF Pd–Rh NANOALLOYS. CRYSTAL STRUCTURE OF Na3[RhOx3]·4H2O. J Struct Chem 62, 782–793 (2021). https://doi.org/10.1134/S0022476621050140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621050140