Abstract
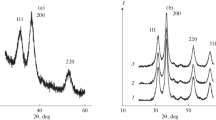
The phase composition of the interaction product of zinc acetate with hydrogen peroxide in a dispersed aqueous phase of a reverse micellar solution based on oxyethylated Brij 30 surfactant and decane as a dispersion medium is analyzed by powder X-ray diffraction (XRD). Along with ZnO2 nanoparticle, the isolated product is found to contain needle-like microcrystals that, according to the single crystal XRD analysis, are zinc lactate dihydrate [Zn(lact)2(H2O)2]: a = 6.0196(3) Å, b = 11.9068(4) Å, c = 14.3878(7) Å, Z = 4, space group P212121. The coordination environment of the Zn atom is a distorted octahedron: Zn–О distances are 2.051(2)-2.099(3) Å, ∠О–Zn–О bond angles are 76.16° and 76.14°. Chelate rings are bent along the О…О line at 22.18° and 11.54°. The Hirshfeld surface (complex sphericity 0.79, asphericity 0.16) is constructed and intermolecular contacts are analyzed. A comparative crystal chemical analysis with the known [Zn(lact)2(H2O)2]·H2O structure is performed.




Similar content being viewed by others
REFERENCES
X. Yu, T. J. Marks, and A. Facchetti. Nat. Mater., 2016, 15, 383-396.
D. Y. Kim. Zinc Oxide Nanostructures for Flexible and Transparent Electronics: PhD Dissertation. Clemson University, 2014.
С. T. Bergs. Syntheses, Characterizations and Applications of Zinc Peroxide Nanoparticles: PhD Dissertation. Aachen University, 2017.
M. Sun, W. Hao, C. Wang, and T. Wang. Chem. Phys. Lett., 2007, 443, 342-346.
L. Rosental-Toib, K. Zohar, M. Alagem, and Y. Tsur. Chem. Eng. J., 2008, 136, 425-429.
H. Bai and X. Liu. Mater. Lett., 2010, 64, 341-343.
R. A. Pawar, D. R. Shinde, and P. S. Tambade. Desalin. Water Treat., 2016, 57, 16514-16521.
F. H. Allen. Acta Crystallogr., Sect. B, 2002, 58(3-1), 380-388.
Powder Diffraction File. PDF-2/Release 2009. International Centre for Diffraction Data: Newtown, PA, 2009.
W. Kraus and G. Nolze. J. Appl. Crystallogr., 1996, 29(3), 301-303.
H. P. Klug and L. E. Alexander. X-Ray Diffraction Procedures. John Wiley & Sons, 1967.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2015, 71, 3-8.
G. M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3-8.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339-341.
S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka, and M. A. Spackman. CrystalExplorer (Version 1.7). University of Western Australia, 2012.
S. I. Dorovskikh, D. A. Piryazev, P. A. Stabnikov, and N. B. Morozova. J. Struct. Chem., 2019, 60(7), 1052-1061.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 4, pp. 611-616.https://doi.org/10.26902/JSC_id71139
Rights and permissions
About this article
Cite this article
Gromilov, S.A., Piryazev, D.A. & Tatarchuk, V.V. CRYSTAL STRUCTURE OF ZINC LACTATE DIHYDRATE – A BY-PRODUCT OF THE MICELLAR SYNTHESIS OF ZnO2 NANOPARTICLES FROM ZINC ACETATE AND HYDROPERITE. J Struct Chem 62, 571–576 (2021). https://doi.org/10.1134/S0022476621040089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621040089