Abstract
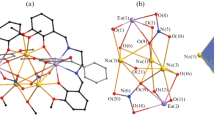
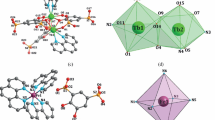
Coordination compounds [Eu{Ph2P(O)Pym}2(NO3)3] (1) and [Tb{Ph2P(O)Pym}2(MeOH)(NO3)3] (2) are synthesized by the interaction of Eu(III) and Tb(III) nitrates with diphenyl(pyrimidin-2-yl)phosphine oxide (Ph2P(O)Pym). According to single crystal XRD data, the crystal structures of 1 and 2 consist of molecules of mononuclear complexes. The Ph2P(O)Pym ligand is bidentate chelating and monodentate in complexes 1 and 2 respectively; coordination polyhedra of the Ln atom are N2O8 in 1 and O9 in 2. The presence of C–H⋯O hydrogen bonds between the neighboring molecules of the complexes leads to the formation of dimeric ensembles organized in chains (in 1) and layers (in 2) by weaker hydrogen bonds. The complexes exhibit metal-centered photoluminescence in the solid state at 300 K with long lifetimes of excited states (τ > 1 ms) and luminescence quantum yields φ = 28% and 14% for complexes 1 and 2 respectively.






Similar content being viewed by others
REFERENCES
A. W. G. Platt. Coord. Chem. Rev., 2017, 340, 62.
Y. Hirai, T. Nakanishi, and Y. Hasegawa. J. Lumin., 2016, 170, 801.
J. C. G. Bünzli. Coord. Chem. Rev., 2015, 293–294, 19.
T. Harada, Y. Hasegawa, Y. Nakano, M. Fujiki, M. Naito, T. Wada, Y. Inoue, and T. Kawai. J. Alloys Compd., 2009, 488, 599.
M. Congiu, M. Alamiry, O. Moudam, S. Ciorba, P. R. Richardson, L. Maron, A. C. Jones, B. S. Richards, and N. Robertson. Dalton Trans., 2013, 42, 13537.
H. Xu, L.-H. Wang, X.-H. Zhu, K. Yin, G.-Yu Zong, X.-Y. Hou, and W. Huang. J. Phys. Chem. B, 2006, 110, 3023.
H. Xu, K. Yin, and W. Huang. Chem. Eur. J., 2007, 13, 10281.
Md. A. Subhan, Y. Hasegawa, T. Suzuki, S. Kaizaki. and Y. Shozo. Inorg. Chim. Acta, 2009, 362, 136.
V. Divya, R. O. Freire, and M. L. P. Reddy. Dalton Trans., 2011, 40, 3257.
D. B. A. Raj, B. Francis, M. L. P. Reddy, R. R. Butorac, V. M. Lynch, and A. H. Cowley. Inorg. Chem., 2010, 49, 9055.
N. E. Borisova, A. V. Kharcheva, S. V. Patsaeva, L. A. Korotkov, S. Bakaev, M. D. Reshetova, K. A. Lyssenko, E. V. Belova, and B. F. Myasoedov. Dalton Trans., 2017, 46, 2238.
S. Mal, M. Pietraszkiewicz, and O. Pietraszkiewicz. J. Coord. Chem., 2015, 68, 367.
S. Biju, N. Gopakumar, J.-C. G. Bünzli, R. Scopelliti, H. K. Kim, M. L. P. Reddy. Inorg. Chem., 2013, 52, 8750.
Y. Hasegawa, R. Hieda, K. Miyata, T. Nakagawa, and T. Kawai. Eur. J. Inorg. Chem., 2011, 32, 4978.
Y. Hirai, T. Nakanishi, K. Miyata, K. Fushimi, and Y. Hasegawa. Mater. Lett., 2014, 130, 91.
K. Miyata, Y. Konno, T. Nakanishi, A. Kobayashi, M. Kato, K. Fushimi, and Y. Hasegawa. Angew. Chem., Int. Ed., 2013, 52, 6413.
K. Miyata, T. Nakanishi, K. Fushimi, and Y. Hasegawa. J. Photochem. Photobiol., A, 2012, 235, 35.
L. J. Charbonniere, R. Ziessel, M. Montalti, L. Prodi, N. Zaccheroni, C. Boehme, and G. Wipff. J. Am. Chem. Soc., 2002, 124, 7779.
S. Xu, M. Liu, H.-L. Han, Z.-F. Li, Q.-H. Jin, W. Su, Y.-Y. Chen, and J.-Y. Yao. Polyhedron, 2015, 85, 69.
T. Harada, Y. Nakano, M. Fujiki, M. Naito, T. Kawai, and Y. Hasegawa. Inorg. Chem., 2009, 48, 11242.
L. R. Melby, N. J. Rose, E. Abranmson, and J. C. Caris. J. Am.Chem. Soc., 1964, 86, 5117.
F. H. Barnes, A. W. Kelly, H. Melzer, H. H. Patterson, and R. D. Pike. Z. Anorg. Allg. Chem., 2018, 644, 525.
Y. Ma, S. Xu, X. Wang, M. Liu, Y.-X. Li, X.-L. Xin, and Q.-H. Jin. Z. Anorg. Allg. Chem., 2017, 643, 780.
D. R. Cousins and F. A. Hart. J. Inorg. Nucl. Chem., 1967, 29, 1745.
R. P. Feazell, J. B. Gary, K. K. Klausmeyer, J. A. Kautz, and C. W. Wong. J. Chem. Crystallogr., 2004, 34, 507.
Y. P. Mascarenhas, R. H. de Almeida Santos, G. R. Leite, and O. A. Serra. J. Inorg. Nucl. Chem., 1973, 35, 2595.
G. Valle, G. Casotto, P. L. Zanonato, and B. Zarli. Polyhedron, 1986, 5, 2093.
W. Levason, E. H. Newman, and M. Webster. Polyhedron, 2000, 19, 2697.
B. G. Vats, S. Kannan, M. Kumar, and M. G. B. Drew. ChemistrySelect, 2017, 2, 3683.
M. T. Reetz, R. Demuth, and R. Goddard. Tetrahedron Lett., 1998, 39, 7089.
CrysAlisPro 1.171.39.46. Rigaku Oxford Diffraction, 2018.
G. M. Sheldrick. Acta Crystallogr., 2015, A71, 3.
G. M. Sheldrick. Acta Crystallogr., 2015, C71, 3.
V. Tsaryuk, V. Zolin, L. Puntus, V. Savchenko, J. Legendziewicz, J. Sokolnicki, and R. Szostak. J. Alloys Compd., 2000, 300–301, 184.
I. R. Ferraro. J. Mol. Spectrosc., 1960, 4, 99.
G. B. Deacon and J. H. S. Green. Spectrochim. Acta, Part A, 1968, 24, 845.
W. T. Carnall and H. Crosswhite. Energy Levels Structure and Transition Probabilities of the Trivalent Lanthanides in LaF3, Argonne. National Laboratory Report. USA, 1977.
Yu. A. Bryleva, L. A. Glinskaya, A. M. Agafontsev, M. I. Rakhmanova, A. S. Bogomyakov, T. S. Sukhikh, E. A. Gorbunova, A. V. Tkachev, and S. V. Larionov. J. Struct. Chem., 2019, 60(8), 1314.
Funding
The work was supported by RFBR within scientific project No. 19-73-00158.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Translated from Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 2, pp. 280-291 https://doi.org/10.26902/JSC_id68351 .
Rights and permissions
About this article
Cite this article
Bryleva, Y.A., Artem’ev, A.V., Glinskaya, L.A. et al. Eu(III) AND Tb(III) COMPLEXES BASED ON DIPHENYL(PYRIMIDIN-2-YL)PHOSPHINE OXIDE: SYNTHESIS, STRUCTURE, AND PHOTOLUMINESCENT PROPERTIES. J Struct Chem 62, 265–276 (2021). https://doi.org/10.1134/S0022476621020116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621020116