Abstract
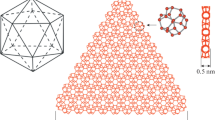
Structural models of stable shells of bound water are built. The models are morphologically similar to the icosahedron and to polyhedral cages within the clathrate water lattice (dodecahedron, Allen′s polyhedra). The bound water shells are built of the primary structural elements (twist-boat derivatives): the 30/11 helix (edges) and K-modules (vertices) of the polyhedral shells. The geometric characteristics of the shells are reported; the volumes of their cages and the structural parameter of the interphase region of bound water are estimated. The work discusses the possibility of applying the structural models of bound water when describing the structure of the interphase region and microheterogeneous properties of water systems when atmospheric gases are dissolved in water. An empirical formula for the surface tension is proposed. The formula contains a structural parameter of the interphase region as well as parameters of the water molecule and those of hydrogen bonding. The model of the surface layer of water is used to suggest a possible molecular mechanism responsible for the formation of “intermediate rotator phases”.










Similar content being viewed by others
Notes
* Allen's polyhedra are polyhedra with 12 pentagonal faces and 0, 2, 3 or 4 hexagonal faces, respectively: dodecahedron 512 and polyhedra 51262, 51263, 51264. Polyhedron 51268 is also included.
* The hydrogen bond parameter RO…O for the frameworks of water clathrates is ~ 0.28 nm.
* The kagome lattice is a tiling (mosaic) composed of regular equilateral triangles and regular hexagons.
REFERENCES
J. D. Bernal and R. H. Fowler. J. Chem. Phys., 1933, 1, 515.
D. Eisenberg and W. Kauzmann. The Structure and Properties of Water. Clarendon: Oxford, 1969.
V. Ya. Antonchenko, A. S. Davydov, and V. V. Il′in. Osnovy Fiziki Vody (Basics of Water Physics) [in Russian]. Naukova Dumka: Kiev, 1991.
G. G. Malenkov. J. Struct. Chem., 2006, 47(Suppl. 1), S1.
O. Ya. Samoilov. Struktura Vodnykh Rastvorov Elektrolitov i Gidrataciya Ionov (The Structure of Aqueous Solutions of Electrolytes and Hydration) [in Russian]. AN SSSR: Moscow, 1957.
Metod Molekulyarnoi Dinamiki v Fizicheskoi Khimii (Method of Molecular Dynamics in Physical Chemistry) [in Russian]. / Ed. Yu. K. Tovbin. Nauka: Moscow:, 1996.
M. P. Allen and D. J. Tildesley. Computer Simulation of Liquids. Oxford University Press, 2017.
I. Z. Fisher. Statistical Theory of Liquids. Chicago Univ., 1964.
G. G. Malenkov, A. V. Teplukhin, and V. I. Poltev. J. Struct. Chem., 1990, 30, 605.
A. Geiger and H. E. Stanley. Phys. Rev., 1982, 49, 1895.
M. N. Rodnikova. J. Phys. Chem., 1993, 67, 275.
M. Rodnikova and J. Barthel. J. Mol. Liq., 2007, 131–132, 121.
M. N. Rodnikova. In: Strukturnaya Samoorganizaciya v Rastvorakh i na Granice Razdela Faz (Structural Self-Organization in Solutions and at the Interface) [in Russian]. LKI: Moscow, 2008, 151–198.
Yu. I. Naberukhin. Strukturnye Modeli Zhidkostei (Structural Models of Liquids) [in Ruissian]. NGU: Novosibirsk, 1981.
N. N. Medvedev. Metod Voronogo-Delone v Issledovanii Struktury Nekristallicheskikh System (Voronoi-Delaunay Method in the Study of the Structure of Non-Crystalline Systems) [in Russian]. SO RAN: Novosibirsk, 2000.
D. L. Tytik. Izv. Ross. Akad. Nauk, Ser. Fiz., 1997, 61, 1743.
V. P. Voloshin, E. A. Zheligovskaya, G. G. Malenkov, Yu. I. Naberukhin, and D. L. Tytik. Russ. Chem. J., 2001, 45, 31.
V. P. Voloshin, Yu. I. Naberukhin, and G. G. Malenkov. Strukt. Din. Mol. Sist. [Online], 2011, 10A, 12, http://old.kpfu.ru/sdms/files10/EJ_S&DMS_10A_p12_25.pdf (accessed 18.06.2020).
G. G. Malenkov and D. L. Tytik. Izv. Ross. Akad. Nauk, Ser. Fiz., 2000, 64, 1469.
G. G. Malenkov, D. L. Tytik, and E. A. Zheligovskaya. J. Mol. Liq., 1999, 82, 27.
G. G. Malenkov, D. L. Tytik, and E. A. Zheligovskaya. J. Mol. Liq., 2003, 106(2–3), 179.
M .N. Rodnikova, S. A. Zasypkin, and G. G. Malenkov. Dokl. Akad. Nauk, 1992. 324, 386.
F. Sciortino, A. Geiger, and H. E. Stanley. Nature, 1991, 354, 218.
G. G. Malenkov, Yu. I. Naberukhin, and V. P. Voloshin. Russ. Chem. J., 2009, 53, 25.
G. G. Malenkov. Colloids Surf., A, 2011, 383, 41.
S. A. Zasypkin and M. N. Rodnikova. J. Phys. Chem., 1993, 67, 323.
D. A. Tanasyuk, A. A. Revina and V. I. Ermakov. High Technol., 2012, 13, 9.
G. H. Pollack. The Fourth Phase of Water. Beyond Solid, Liquid, and Vapor. Ebner and Sons, 2013.
N. F. Bunkin, V. A Kozlov, A. V. Shkirin, B. W. Ninham, A. A. Balashov, and S. V. Gudkov. J. Chem. Phys., 2018, 148, 124901-1-7.
S. P. Gabuda. Svyazannaya Voda. Fakty i Gipotezy (Bound Water. Facts and Hypotheses) [in Russian]. Nauka SO: Novosibirsk, 1982.
Yu. A. Dyadin, K. A. Udachin, and I. V. Bondaryuk. Soedineniya vklyucheniya (Inclusion Compounds) [in Russian]. NGU: Novosibirsk, 1988.
V. P. Belosludov, Yu. A. Dyadin, and M. Yu. Lavrent′ev. Teoreticheskie Modeli Klatratoobrazovaniya (Theoretical Models of Clathrate Formation) [in Russian]. Nauka: Novosibirsk, 1991.
A. Y. Manakov, V. I. Kosyakov, and S. F. Solodovnikov. Structural Chemistry of Clathrate Hydrates and Related Compounds. In: Comprehensive Supramolecular Chemistry II. Vol. 7: Supramolecular Engineering: Designing the Solid State / Ed. J. L. Atwood. Elsevier, 2017, 161–206.
A. Thorpe, A. R. Stubbs, A. J. Hall, and R. J. Turner. Nature, 1982, 296, 636.
N. F. Bunkin, O. I. Vinogradova, A. I. Kuklin, A. V. Lobeev, and T. G. Movchan. JETP Lett., 1995, 62, 685.
S. H. Oh and J.-M. Kim. Langmuir, 2017, 33, 3818.
N. F. Bunkin and A. V. Shkirin. Tr. Inst. Obshch. Fiz. im. A. M. Prokhorova, Ross. Akad. Nauk, 2013, 69, 3.
H. Kobayashi, S. Maeda, M. Kashiwa, and T. Fujita. Proc. of SPIE, 2014, 9232, 92320U-1.
T. Fujita. The Status and Future of Fine Bubble Generation, Measurements and Applications. Presentation of the report. 7th International Symposium of Fine Bubble Technology. Sydney, Australia. July 25, 2016. https://www.standards.org.au/StandardAU/Media/SA-Archive/OurOrganisation/Events/Documents/2-Dr-Toshihiro-Fujita,-Vice-Chairman-of-FBIA.pdf (accessed 18.06.2020).
D. L. Tytik, S. A. Busev, V. V. Vysotskii, A. A. Revina, O. V. Souvorova, V. I. Kuz′min, and A. F. Gadzaov. Russ. J. Phys. Chem. A, 2019, 93, 2502.
L. Pauling and P. Pauling. Chemistry. W.H. Freeman and Co (Sd), 1975.
N. A. Bulienkov and E. A. Zheligovskaya. Russ. J. Phys. Chem., 2006, 80, 1584.
N. Denkov, S. Tcholakova, I. Lesov, D. Cholakova, and S. K. Smoukov. Nature, 2015, 528, 392.
A. H. Boerdijk. Philips Res. Rep., 1952, 7, 303.
H. S. M. Coxeter. Regular Polytopes. Macmillan, 1963.
N. A. Bul′enkov. Kristallografiya, 1988, 33, 424.
N. A. Bul′enkov. Sov. Phys. Crystallogr., 1990, 35, 88.
N. A. Bulienkov. Biofizika, 1991, 36, 181.
N. A. Bulienkov. Three Possible Branches of Determinate Modular Generalization of Crystallography. In: Fields Institute Monographs. Vol. 10: Quasicrystals and Discrete Geometry / Ed. J. Patera. Providence, Rhode Island: Am. Math. Soc., 1998, 67.
N. A. Bulienkov. Biofizika, 2005, 50, 620.
N. A. Bulienkov. Crystallography, 2011, 56, 729.
N. A. Bulienkov and E. A. Zheligovskaya. Struct. Chem., 2017, 28, 75.
E. A. Zheligovskaya and G. G. Malenkov. Russ. Chem. Rev., 2006, 75, 57.
J. G. Vinter, A. Davis, and M. R. Saunders. J. Comput.-Aided Mol. Des., 1987, 1, 31.
M. Kornfeld and L. Suvorov. J. Appl. Phys., 1944, 15, 495.
M. Kornfeld. Uprugost i Prochnost Zhidkostei (Elasticity and Strength of Liquids) [in Russian]. GITTL: Moscow–Leningrad, 1951.
N. Denkov, D. Cholakova, S. Tcholakova, and S. K. Smoukov. Langmuir, 2016, 32, 7985.
D. Cholakova, N. Denkov, S. Tcholakova, I. Lesov, and S. K. Smoukov. Adv. Colloid Interface Sci., 2016, 235, 90.
D. Cholakova, Z. Valkova, S. Tcholakova, N. Denkov, and S. Smoukov. Langmuir, 2017, 33, 5696.
P. A. Haas, R. E. Goldstein, S. K. Smoukov, D. Cholakova, and N. Denkov. Phys. Rev. Lett., 2017, 118, 088001.
D. Cholakova and N. Denkov. Adv. Colloid Interface Sci., 2019, 269, 7.
S. R. Liber, O. Marin, A. V. Butenko, R. Ron, L. Shool, A. Salomon, M. Deutsch, and E. Sloutskin. J. Am. Chem. Soc., 2020, 142, 8672.
Yu. K. Tovbin. Malyye Sistemy I Osnovy Termodinamiki. (Small Systems and the Basics of Thermodynamics) [in Russian]. Fizmatlit: Moscow, 2018.
B. D. Summ. Russ. J. Phys. Chem., 2005, 79, 141.
B. D. Summ. Vestn. Mosk. Univ., Ser. 2: Khim., 1993, 34, 59.
I. Syozi. Progr. Theor. Phys., 1951, VI, 306.
C. Broholm, G. Aeppli, G. P. Espinosa, and A. S. Cooper. Phys. Rev. Lett., 1990, 65, 3173.
H. S. M. Coxeter. Introduction to Geometry. Wiley: New York, 1961.
G. Némety and H. A. Scheraga. J. Chem. Phys., 1962, 36, 3382.
O.V. Konovalov. Kristallograficheski Pravil′Nye Razbieniya Evklidova Prostranstva na Polupravil′Nye Izgony (Crystallographically Correct Partitions of Euclidean Space into Semi-Regular Isogons) [in Russian]. Preprint No. 7. IKAN USSR: Moscow, 1988.
A. V. Shevelkov. Vestn. Mosk. Univ., Ser. 2: Khim., 2003, 44, 163.
V. V. Novikov, K. S. Pilipenko, A. V. Matovnikov, N. V. Mitroshenkov, M. S. Likhanov, A. S. Tyablikov, and A. V. Shevelkov. Dalton Trans., 2018, 47, 11219.
B. V. Deryagin, N. V. Churaev, and V. M. Muller. Poverkhnostnye Sily (Surface Forces) [in Russian]. Nauka: Moscow, 1985.
J. N. Israelachvili and R. M. Pashley. Nature, 1983, 306, 249.
J. N. Israelachvili. Intermolecular and Surface Forces. Academic Press: Cambridge, 2011.
A. D. Alexandrov. Vypuklye Mnogogranniki (Convex Polyhedra) [in Russian]. GITTL: Moscow–Leningrad, 1950.
Funding
The reported study was partially funded by RFBR, project number 19-03-00696.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflict of interests.
Additional information
Translated from Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 2, pp. 219-234 https://doi.org/10.26902/JSC_id68317 .
Rights and permissions
About this article
Cite this article
Tytik, D.L. SIMULATION OF HYDRATION SHELLS OF GAS NANOBUBBLES DISSOLVED IN WATER. J Struct Chem 62, 206–220 (2021). https://doi.org/10.1134/S0022476621020049
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621020049