Abstract
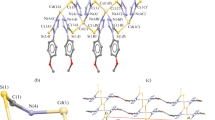
A series of reactions between an oxazolidine ligand 2-(2-(pyridin-2-yl)oxazolidin-3-yl)-N-(pyridin-2-ylmethylene)ethanamine (POPME) and CdX2 (X = Cl, Br, I) salts with different M:L molar ratios (1:1 and 2:1) is experimentally studied to determine the most stable product of these reactions. All products are characterized by the elemental analysis, FTIR, Raman, and 1H NMR spectroscopy, and single crystal X-ray diffraction. Examinations reveal that only one product is formed from 1:1 and 2:1 M:L reactions between each cadmium(II) halide with POPME. The thermodynamic stability and charge distribution patterns of the compound containing bromide are analyzed by DFT and NBO.
Similar content being viewed by others
References
K. Li, H. Chen, Y. Wang, Z. Shan, J. Yang, and P. Brutto. J. Cleaner Prod., 2009, 17, 1603.
W. Kang, W. Wang, X. Zhi, B. Zhang, P. Wei, and H. Xu. Bioorg. Med. Chem. Lett., 2012, 22, 1187.
R. L. Dow, B. M. Bechle, T. T. Chou, D. A. Clark, B. Hulin, and R. W. Stevenson. J. Med. Chem., 1991, 34, 1538.
S. L. Shapiro, I. M. Rose, F. C. Testa, E. Roskin, and L. Freedman. J. Am. Chem. Soc., 1959, 81, 6498.
S.-Y. Na and H.-J. Kim. Dyes Pigm., 2016, 134, 526.
S. G. Davies and H. J. Sanganee. Tetrahedron: Asymmetry, 1995, 6, 671.
T. N. Le, Q. P. B. Nguyen, J. N. Kim, and T. H. Kim. Tetrahedron Lett., 2007, 48, 7834.
F. Huguenot and T. Brigaud. J. Org. Chem., 2006, 71, 7075.
G. Chaume, M.-C. Van Severen, L. Ricard, and T. Brigaud. J. Fluor. Chem., 2008, 129, 1104.
R. S. B. Gonçalves, C. R. Kaiser, M. C. S. Lourenço, M. V. N. D. Souza, J. L. Wardell, S. M. S. V. Wardell, and A. D. D. Silva. Eur. J. Med. Chem., 2010, 45, 6095.
O. Merino, B. M. Santoyo, L. E. Montiel, H. A. Jiménez-Vázquez, L. G. Zepeda, and J. Tamariz. Tetrahedron, 2010, 51, 3738.
N. Gandhi, B. K. Srivastava, V. B. Lohray, and B. B. Lohray. Tetrahedron, 2004, 45, 6269.
M. Mohammadizadeh and N. Firoozi. Tetrahedron, 2010, 51, 2467.
T. E. Kokina, L. A. Glinskaya, E. S. Vasiliev, M. I. Rakhmanova, S. V. Makarova, D. A. Piryazev, I. V. Korol’kov, A. V. Tkachev, and S. V. Larionov. J. Struct. Chem., 2017, 58, 994.
V. V. Kovalev, Y. E. Gorbunova, G. A. Razgonyaeva, and Y. V. Kokunov. Russ. J. Inorg. Chem., 2015, 60, 1487.
N. A. Bogachev, G. L. Starova, A. V. Razzhivin, M. Y. Skripkin, and A. B. Nikolskii. Russ. J. Gen. Chem., 2018, 88, 1.
Y. V. Kokunov, V. V. Kovalev, Y. E. Gorbunova, and G. A. Razgonyaeva. Russ. J. Coord. Chem., 2015, 41, 787.
N. S. Rukk, L. G. Kuzmina, D. V. Albov, R. S. Shamsiev, S. N. Mudretsova, G. A. Davydova, V. M. Retivov, P. A. Volkov, V. V. Kravchenko, G. N. Apryshko, A. N. Streletskii, A. Y. Skryabina, E. A. Mironova, and V. V. Zamalyutin. Polyhedron, 2015, 102, 152.
I. S. Ivanova, I. N. Polyakova, E. S. Krivorot’ko, E. N. Pyatova, V. E. Baulin, and A. Y. Tsivadze. Russ. J. Inorg. Chem., 2017, 62, 1299.
Y. V. Kokunov, V. V. Kovalev, Y. E. Gorbunova, G. A. Razgonyaeva, and S. A. Kozyukhin. Russ. J. Inorg. Chem., 2018, 63, 333.
M. Hakimi, Z. Mardani, K. Moeini, M. Minoura, and H. Raissi. Z. Naturforsch., 2011, 66b, 1122.
Z. Mardani, M. Hakimi, K. Moeini, and F. Mohr. Acta Crystallogr., Sect. C, 2019, 951.
G. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112.
G. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339.
L. J. Farrugia. J. Appl. Crystallogr., 1997, 30, 565.
G. Bergerhoff, M. Berndt, and K. Brandenburg. J. Res. Natl. Inst. Stand. Technol., 1996, 101, 221.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian 09. Wallingford, CT, USA: Gaussian, 2009.
J. P. Perdew. Phys. Rev., 1986, B33, 8822.
A. D. Becke. J. Chem. Phys., 1993, 98, 5648.
R. Ditchfield, W. J. Hehre, and J. A. Pople. J. Chem. Phys., 1971, 54, 724.
P. J. Hay and W. R. Wadt. J. Chem. Phys., 1985, 82, 270.
G. Abbas, A. Hassan, A. Irfan, M. Mir, R. M. Al, and G. Wu. J. Struct. Chem., 2015, 56, 92.
M. Ghiasi, S. Kamalinahad, and M. Zahedi. J. Struct. Chem., 2014, 55, 1574.
M. Hakimi, Z. Mardani, K. Moeini, and F. Mohr. Polyhedron, 2015, 102, 569.
K. Nakamoto. Infrared and Raman Spectra of Inorganic and Coordination Compounds. John Wiley: Hoboken, 2009).
F. H. Allen. Acta Crystallogr., Sect. B, 2002, 58, 380–388.
Z. Mardani, V. Golsanamlou, S. Khodavandegar, K. Moeini, A. M. Z. Slawin, and J. D. Woollins. J. Coord. Chem., 2018, 71, 120.
M. Hakimi, Z. Mardani, K. Moeini, N. Feizi, and F. Mohr. J. Coord. Chem., 2017, 70, 1247.
F. Marandi, K. Moeini, B. Mostafazadeh, and H. Krautscheid. Polyhedron, 2017, 133, 146.
F. Marandi, K. Moeini, S. Ghasemzadeh, Z. Mardani, C. K. Quah, and W.-S. Loh. J. Mol. Struct., 2017, 1149, 92.
C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. Mccabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. Van De Streek, and P. A. Wood. J. Appl. Crystallogr., 2008, 41, 466.
A. Gavezzotti and G. Filippini. J. Phys. Chem., 1994, 98, 4831.
A. Gavezzotti. Acc. Chem. Res., 1994, 27, 309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflict of interests.
Additional information
Additional Information
CCDC 939155 for 2mono contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB21EZ, UK; fax: +44(0)1223-336033; email: deposit@ccdc.cam.ac.uk). Structure factor table is available from the authors.
Text © The Author(s), 2020, published in Zhurnal Strukturnoi Khimii, 2020, Vol. 61, No. 6, pp. 904–912.
Rights and permissions
About this article
Cite this article
Mardani, Z. Coordination of a (Npy)2NimineNamine-Donor Oxazolidine Ligand Toward CdX2 (X = Cl, Br, I) with 1:1 and 2:1 M:L Molar Ratios Along with Spectral, Theoretical, and Structural Studies. J Struct Chem 61, 852–860 (2020). https://doi.org/10.1134/S0022476620060037
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476620060037